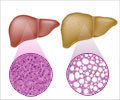
Genetic mechanism behind liver cancer's link to fat metabolism disruption.

Genetic screening of liver cancer: State of the art
Go to source). The liver serves as the body’s metabolic hub, maintaining a precise balance between fat synthesis and breakdown. In healthy individuals, this balance is tightly regulated. However, in hepatocellular carcinoma (HCC), liver cells accumulate abnormal levels of fat droplets, which fuel unchecked tumour growth. This lipid buildup is primarily driven by the mTORC1 signaling pathway—a molecular switch that activates lipogenic genes—and SREBP proteins, the master regulators of fat metabolism. In cancerous cells, mTORC1 becomes hyperactive, accelerating fat production and triggering a self-sustaining cycle that promotes tumour progression.
TOP INSIGHT
Did You Know?
Changes in genes that regulate cell growth, division, or DNA repair can contribute to #livercancer development. #medindia #cancergenetics #liverdisease #geneticpredisposition
Unrevealing the Mystery: Fat and Liver Cancer
The liver is the body’s metabolic powerhouse, responsible for balancing fat production and breakdown. In healthy individuals, this process is tightly controlled. However, in HCC, liver cells accumulate excessive fat droplets, fuelling tumours to grow uncontrollably. This fat buildup is largely controlled by a key cellular pathway known as mTORC1, a molecular switch that activates fat-producing genes, and by SREBP proteins, which act as master regulators that directly control enzymes involved in fat synthesis. In cancer cells, mTORC1 becomes hyperactive, ramping up fat production and creating a vicious cycle that promotes tumour growth.Genetic Insights: VPS72's Impact on Liver Cancer
The amplification is linked to poorer survival rates, suggesting that this genetic player plays a central role in cancer progression. Here is how the mechanism works:
- DNA Packaging: VPS72 helps attach specific proteins to DNA, which affects whether certain genes are turned on or off.
- Suppressing Protective Genes: VPS72 adds chemical tags to the promoter of gene ATF3, which normally helps prevent cancer growth. This tagging shuts down the production of ATF3, leading to the overactivity of a cancer-promoting pathway called mTORC1.
- Increasing Fat Production: When mTORC1 is overactive, it boosts proteins that increase the production of fats. This results in cancer cells being flooded with fats, providing them with energy and materials needed to grow rapidly.
A Promising Target for Liver Cancer Therapy
These findings offer new hope for targeted therapies. One approach is to design drugs that block VPS72's interaction with H2AZ proteins, potentially stopping the DNA changes that lead to cancer. Another strategy involves using existing drugs that inhibit mTORC1, a pathway already targeted in other cancers, which could be repurposed for liver cancer patients with increased VPS72 activity. By focusing on VPS72 and the pathways it affects, Professor Zhang’s team hopes to stop cancer from progressing.
"Our research shows that the gene VPS72 plays two key roles in liver cancer (HCC): it affects both how genes are controlled and how fat metabolism goes wrong. The findings help explain how liver cancer develops and suggest new ways to treat cancers driven by abnormal fat metabolism,” said Professor Jiangwen Zhang, corresponding author of the study. This research uncovers a critical link between genetic regulation, fat metabolism, and liver cancer.
By targeting the genetic player VPS72 and its cancer-promoting pathway, scientists hope to open the door to more precise and effective treatments—potentially turning off a key fuel supply that liver tumours depend on.
Reference:
- Genetic screening of liver cancer: State of the art - (https://pmc.ncbi.nlm.nih.gov/articles/PMC11135278/)
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email