Moxetumomab pasudotox-tdfk Medication Information
Get detailed information on Moxetumomab pasudotox-tdfk, including pronunciation, uses, dosage guidelines, indications, and instructions on how and when to take it and when to avoid it.
The updated prescription information on Moxetumomab pasudotox-tdfk provides an overview of possible side effects, precautions, warnings, and storage tips.
You'll also find brand names used in india and internationally, along with pricing details. For further clarification, consult your healthcare provider.
Generic Name : Moxetumomab pasudotox-tdfk Pronunciation : mox-e-TOOM-oh-mab pa-SOO-doe-tox tdfk Therapeutic Classification : ChemotherapyTrade Names/Brand Names of Moxetumomab pasudotox-tdfk
India :
Lumoxiti
Why is Moxetumomab pasudotox-tdfk Prescribed? (Indications)
Moxetumomab pasudotox-tdfk is prescribed to treat Hairy Cell Leukemia (HCL) in adult patients who had treatment failure with at least two treatments or whose cancer returned after chemotherapy.Moxetumomab pasudotox-tdfk has a specific protein linked with a toxin that delivers the toxic substance directly into the cancer cells and kills or stops the cancer growth.
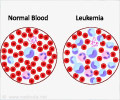
Hairy cell leukemia is a slow-growing, and a rare type of cancer in which the bone marrow produces abnormal B cells, a type of white blood cell (WBC) known as lymphocytes resulting in decreased levels of red blood cells, WBC, and platelets.
HCL affects people aged between 40 and 60 and is common in men compared to women.
When should Moxetumomab pasudotox-tdfk not be taken? (Contraindications)
Moxetumomab pasudotox-tdfk should not be used in patients-• If there is an allergy to moxetumomab pasudotox
• Pregnancy and breastfeeding
• History of severe kidney disease
Children under 18 years of age should not be given moxetumomab pasudotox-tdfk due to lack of safety and effectiveness data in these groups.
What is the dosage of Moxetumomab pasudotox-tdfk?
• The dose of moxetumomab pasudotox-tdfk is calculated based on the individual patient’s body weight.• The recommended dose of moxetumomab pasudotox-tdfk is 0.04 mg/kg given very slowly over 30 minutes on days 1, 3, and 5 of every treatment cycle (28-days).
• The treatment with moxetumomab pasudotox-tdfk should be continued for a maximum of 6 cycles.
• Moxetumomab pasudotox-tdfk can be given until the patient shows any progression of the disease or until the treatment with the drug does not result in intolerable toxicity.
How should Moxetumomab pasudotox-tdfk be taken?
Moxetumomab pasudotox-tdfk is available as a liquid which should be given very slowly intravenously (IV) or deep into the vein as an infusion.Patients should be intravenously given 1 liter of isotonic fluids such as 0.9% sodium chloride or 5% dextrose for 2 to 4 hours before and after each moxetumomab pasudotox-tdfk infusion.
Moxetumomab pasudotox-tdfk should be mixed or diluted under a controlled environment free from pathogens. Add IV solution stabilizer to the mixing bag before adding moxetumomab pasudotox-tdfk for uninterrupted flow.
Gently invert the IV bag for mixing. Do not shake. The diluted solution must be used immediately. If kept in cold storage, it should be allowed to come to room temperature before infusing but not more than 4 hours.
In patients with low body weight (below 50 kg), only half liter of hydration with isotonic fluids is recommended.
Patients who have a history of blood clots should take a low dose of aspirin from day 1 to 8 in a 28-day treatment cycle.
Patients must be given certain medications before and after the moxetumomab pasudotox-tdfk to reduce the severity of the side effects.
Premedications given 30 to 90 minutes before moxetumomab pasudotox-tdfk infusion include-
• Medicine for allergies (e.g., Diphenhydramine or Hydroxyzine)
• Fever medication (e.g., Paracetamol or Acetaminophen)
• Ulcer healing agents (e.g., Ranitidine or Famotidine)
Steroid taken by mouth or given as an injection is advised half an hour before starting the moxetumomab pasudotox-tdfk infusion.
Medications to be given after moxetumomab pasudotox-tdfk infusion:
• Allergy or fever-reducing pills taken by mouth for up to 24 hours after the infusion
• Steroids (e.g. Dexamethasone 4 mg) taken by mouth to reduce nausea and vomiting
Patients must be provided with adequate water or oral fluids (milk or juice) intake after the moxetumomab pasudotox-tdfk infusion from day 1 to 8 in a treatment cycle - patients with a healthy weight get 3 liters and patients who are below 50 kg get 2 liters.
What are the warnings and precautions for Moxetumomab pasudotox-tdfk?
• Patients should be monitored for changes in weight and blood pressure before each moxetumomab pasudotox-tdfk infusion. They should be carefully observed and treated for any symptoms or signs of capillary leak syndrome (CLS) such as weight gain, low blood pressure, fluid accumulation, and breathing difficulty.• Blood and urine parameters should be checked before each dose of moxetumomab pasudotox-tdfk and on day 8 of every treatment cycle. Patients need medical supervision as they can develop hemolytic uremic syndrome with anemia, low platelet count, and high levels of creatinine and bilirubin.
• Kidney function and changes in urine output should be monitored before each infusion and at regular intervals throughout the treatment.
• Infusion-related reactions such as fever, headache, and vomiting may occur during the treatment with the moxetumomab pasudotox-tdfk which can be managed by appropriate premedications and steroids.
• Electrolyte abnormalities with muscle cramps and unusually low calcium levels are expected during the treatment cycle. Therefore monitor the electrolyte level before each dose and on day 8 of every treatment cycle.
What are the side effects of Moxetumomab pasudotox-tdfk?
Gastrointestinal: Hard stools, nausea, vomiting, diarrheaNervous system: Headache, dizziness, fever, abnormal weakness
Lab abnormalities: Low levels of red blood cells, neutrophils, and platelets, high levels of creatinine and liver enzymes, decreased levels of albumin, calcium, sodium, potassium, magnesium, and phosphate
Eye: Blurred vision, dry eye, pain and swelling of the eye, eye discharge, cataract
Others: Fluid accumulation, weight gain, chills
What are the other precautions for Moxetumomab pasudotox-tdfk?
• Females of reproductive potential should use effective contraception during moxetumomab pasudotox-tdfk treatment and should continue the contraception for at least 30 days after the last dose of moxetumomab pasudotox-tdfk.• It is also necessary to verify the pregnancy status of female patients to avoid the risk caused to unborn babies.
• In case of moderate side effects, the treatment can be stopped temporarily until the symptoms are resolved; if the side effects are severe or life-threatening, the treatment can be permanently discontinued.
What are the Drug Interactions of Moxetumomab pasudotox-tdfk?
The doctor should be informed before about any medications or herbal supplements that the patient is taking or is planning to take to avoid life-threatening side effects caused due to interactions of moxetumomab pasudotox-tdfk with other drugs.What are the storage conditions for Moxetumomab pasudotox-tdfk?
• Store the moxetumomab pasudotox-tdfk vial and the IV solution stabilizer in a refrigerator at 2°C to 8°C.• The vials should be placed in the original carton and must be protected from light.
• The diluted solution of moxetumomab pasudotox-tdfk should be used within 4 hours if kept at room temperature between 20°C and 25°C.
• If kept in a refrigerator at 2°C to 8°C, the diluted solution must be used within 24 hours.
• Do not shake or freeze the vial or the diluted solution of moxetumomab pasudotox-tdfk.