The diverse origins of cells that drive fibrosis, that scars and ultimately destroys organs such as the lungs, liver and kidneys were tracked down and quantified by scientists.
"Answering a fundamental question about the origin of these cells by identifying four separate pathways involved in their formation allows us to look at ways to block those pathways to treat fibrosis," said senior author Raghu Kalluri, Ph.D., M.D., MD Anderson chair and professor of Cancer Biology. "It's highly unlikely that a single drug will work."
"In addition to being lethal in its own right, fibrosis is a precursor for the development of cancer and plays a role in progression, metastasis and treatment resistance," Kalluri said. "In some cancers, such as pancreatic cancer, up to 95 percent of tumors consist of fibrotic stroma."
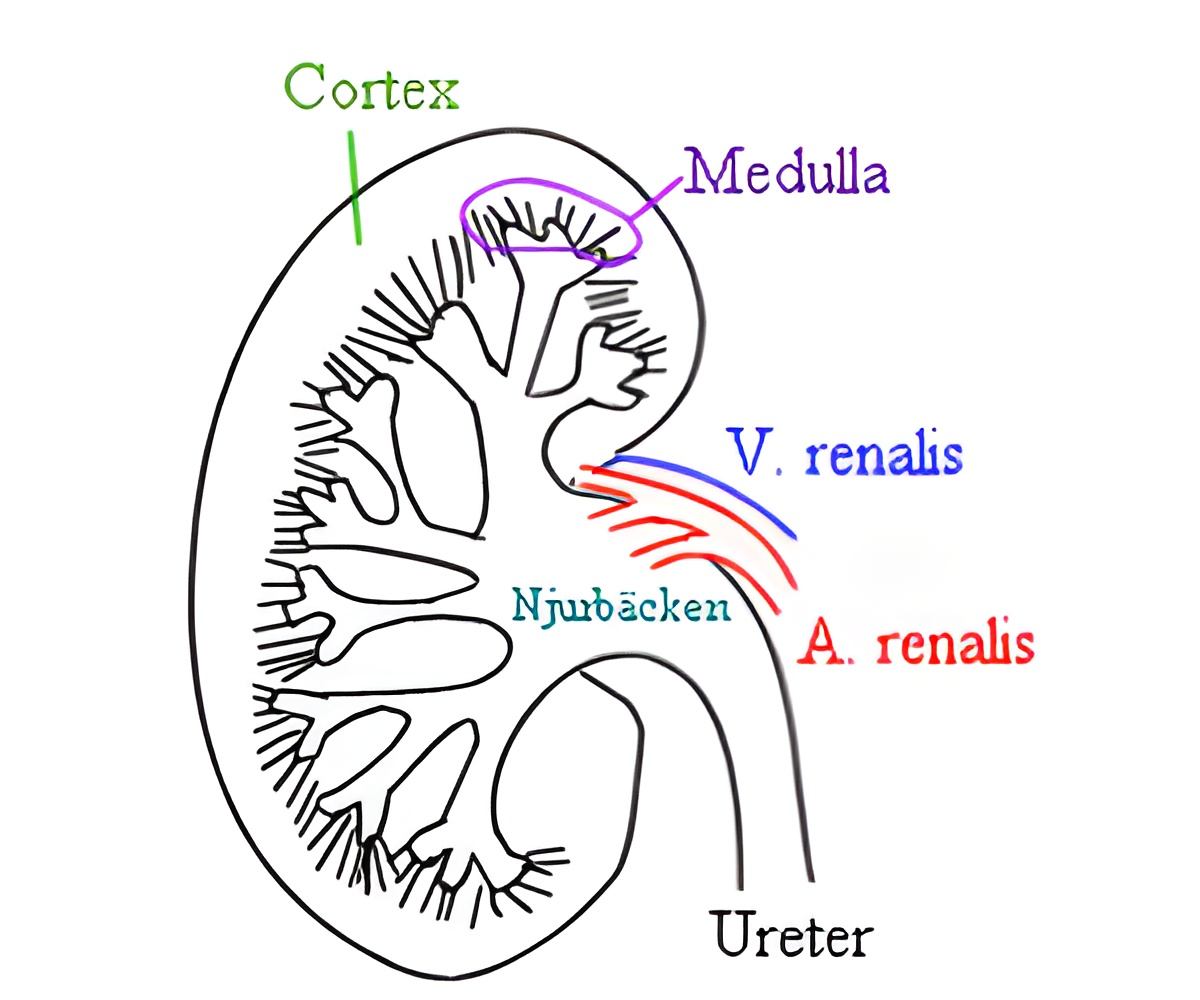
Working in genetic mouse models of kidney fibrosis, Kalluri and colleagues identified four sources of cells called myofibroblasts, the dominant producers of collagen. Collagen normally connects damaged tissue and serves as scaffolding for wound-healing. As healing occurs, myofibroblasts and collagen usually diminish or disappear.
In fibrosis, collagen production marches on. While inflammation-inhibiting drugs can sometimes slow its progress, fibrosis now is treatable only by organ transplant.
Myofibroblasts have four types of parents
This was particularly important because two of the four sources of myofibroblasts start out as another cell type and differentiate into the collagen-producing cells.
- Half of all myofibroblasts are produced by the proliferation of pre-existing resting fibroblasts.
- Another 35 percent are produced by mesenchymal stem cells that originate in the bone marrow, migrate to the "wound" site, and then differentiate into myofibroblasts.
- An additional 10 percent are the products of endothelial to mesenchymal transition (EndMT), in which blood vessel cells change into mesenchymal cells, then become myofibroblasts.
- The final 5 percent come from epithelial to mesenchymal transition (EMT), in which functional cells of an organ sometimes behave like mesenchymal cells and myofibroblasts.
"These differentiation pathways provide leads for drug targets," Kalluri said. "Combining an antiproliferation drug with therapies that block one or more differentiation pathways could provide a double hit to control fibrosis. We hope to synergize these pathways for the most effective therapeutic response."
Recruitment from the bone marrow, EMT and EndMT appear to rely on transforming growth factor beta 1 (TGF-B1) to differentiate into myofibroblasts.
Pericytes are not involved
Some earlier descriptive studies implicated pericytes – connective, contractile cells that surround blood vessels – in the creation of myofibroblasts. The researchers tested pericytes via fate-mapping and found that they're not involved in myofibroblast generation.
Deleting pericytes did not improve kidney fibrosis or change the recruitment of myofibroblasts.
While their research focused on kidney fibrosis, the scientists believe their findings will be applicable to other types of fibrosis.
"Recruitment of fibroblasts is heterogonous. The sources are likely to be the same for lung or liver fibrosis, but the ratios may be different," Kalluri said. "Now we need to go into those other organs and establish a baseline of what we're facing like we did in kidney fibrosis."
Kalluri holds the Rebecca Meyer Brown and Joseph Mellinger Brown Chair in Basic Science Research and also and directs MD Anderson's Metastasis Research Center.
Source-Eurekalert
 MEDINDIA
MEDINDIA



 Email
Email