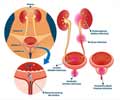
3D 'mini bladders' aid in unraveling pathogen-host dynamics, advancing urinary tract infections’ treatment insights for effective interventions.

A human urothelial microtissue model reveals shared colonization and survival strategies between uropathogens and commensals
Go to source).
’Mini Bladders’ Unveil the Bug-Host Connection in Urinary Tract Infection
Urinary tract infection (UTI) is a growing problem, with around 400 million global cases per year and an estimated 250,000 UTI-related deaths associated with antimicrobial resistance (AMR). Although UTI is often perceived as a simple bacterial infection, 25-30% of UTIs recur within six months despite antibiotic therapy for reasons that are poorly understood.‘Artificial bladder study could help women to receive effective diagnosis and treatment for urinary tract infections. #minibladder #hostbuglink #uti #urinaryinfection #kidneyhealth’





A condition that primarily affects women, UTI has been historically understudied and underfunded, with no improved anti-infective treatments introduced since Alexander Fleming discovered antibiotics nearly a century ago. Diagnosis primarily rests on the midstream urine culture method (dipstick test), an early 20th-century technique that is known to miss many infections.The study suggests that the ‘one size fits all’ approach to diagnosis and treatment currently used in most healthcare systems is inadequate.
These ‘mini bladders’ were exposed to six bacterial species commonly found in the human bladder: Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Proteus mirabilis, Streptococcus agalactiae and Klebsiella pneumoniae.
Professor Jennifer Rohn, senior author of the study from UCL Division of Medicine, said: “We put a variety of UTI bacteria species and strains through their paces and discovered a battleground of diversity. One of the key observations was the importance of persistence. If you want to be a successful pathogen, you have to have strategies that help you to survive treatment and hide from patrolling immune cells, which means you live to fight another day".
“Some species of both ‘good’ and ‘bad’ bugs formed pods within the bladder wall, most likely as a way of surviving in this harsh environment. If this happens with a friendly bug, this isn’t a problem. But if the bug is causing an infection, this poses a serious problem for diagnosis and treatment because the bacteria aren’t necessarily going to be detected in a urine sample or be in a position where oral antibiotics can reach them.”
Advertisement
Dr. Carlos Flores, first author of the study from UCL Division of Medicine, said: “Based on our results, next-generation diagnostics for UTIs could focus on identifying ‘bad’ bugs based on how the body responds, rather than trying to spot the presence of problem bacteria among the background noise of the microbiome. There are so many species and strains of bacteria in the human bladder that we don’t fully understand, but the body seems to be pretty good at telling friend from foe.”
Advertisement
Professor Rohn concluded: “This study confirms what many women who’ve struggled with persistent UTIs already know, which is that the current methods of diagnosing and treating these infections are inadequate".
“Urine dipstick tests are too likely to miss infections hiding in the bladder wall, especially when a patient’s first response to discomfort is to drink lots of water, which dilutes the test. Not all bugs can be cultured in the lab, and even if they could be that doesn’t tell us if this strain is the cause of an infection or if its position in the bladder wall would make the standard three-day course of antibiotics unlikely to eradicate it.”
Helen Lucas, a patient from Hertfordshire who has struggled with chronic UTIs for several years, said: “Before my UTI problem started, I just assumed that living in the UK you could go to your doctor, be prescribed treatment, and get better. It’s been a shock to find that the testing and treatment of UTIs, even the perceptions of what this disease can be, are so antiquated".
“If I wasn’t outspoken, I think might have been left with an undiagnosed chronic UTI, unable to fully live my life. I know other people are struggling to overcome the same hurdles that I had to. It’s fantastic that research is being done into why UTIs persist, which will hopefully lead to better testing and treatment.”
Reference:
- A human urothelial microtissue model reveals shared colonization and survival strategies between uropathogens and commensals - (https://www.science.org/doi/10.1126/sciadv.adi9834)
Source-Eurekalert