Specific compound that may help prevent the risk of a form of arrhythmia that occurs from common medications.
TOP INSIGHT
Multi-institutional team combined expertise make a discovery of the compound that may help prevent the risk of a form of arrhythmia that occurs from common medications. It was found that the compound, C28 could prevent or reverse the drug-induced prolongation of the electrical signals across the cardiac cell membrane and minimally affected the normal action potentials at the same dosage with no significant effects.
Several drugs that are known to cause a prolongation of the QT interval of the heartbeat, that predisposes patients to cardiac arrhythmia and sudden death, have been pulled from the market.
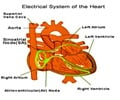
Long QT also can be caused by specific mutations in genes that code for ion channel proteins, which conduct the ionic currents to generate the action potential in rare cases. There are several types of ion channels in the heart where a change in one or more of them may lead to arrhythmia.
Arrhythmias Prevented by a Specific Compound
The team selected a specific target, IKs – one of the two potassium channels that are activated and play a major role during the action potential (IKr (rapid) and IKs (slow)) for their work. Since IKr (rapid) plays a major role in the action potential, blocking it would result in long QT intervals. On the contrary, IKs are very slow and contribute much less to the normal action potential duration.
By analyzing the geometric and chemical traits of the small compounds, the team was able to identify C28 that holds the potential to enhance the IKs channel function. They also studied a series of genetically modified IKs channels to reveal the binding of C28 to the site for the in silico screening.
"We are very excited about this. In many of these medications, there is a concentration of the drug that is acceptable, and at higher doses, it becomes dangerous. If C28 can eliminate the danger of inducing Q-T prolongation, then these drugs can be used at higher concentrations, and in many cases, they can become more therapeutic," says Ira Cohen, MD, Ph.D., Distinguished Professor of Physiology and Biophysics, professor of medicine, and director of the Institute for Molecular Cardiology at the Renaissance School of Medicine at Stony Brook University.
However, the study also affirmed that the compound needs additional verification and testing to prove its efficacy.
"This work was done by an effective drug design approach: identifying a critical site in the ion channel based on the understanding of structure-function relation, using in silico docking to identify compounds that interact with the critical site in the ion channel, validating functional modulation of the ion channel by the compound, and demonstrating therapeutic potential in cardiac myocytes. Our three labs form a great team, and without any of them, this would not be possible," says, Xiaoqin Zou, professor of physics, biochemistry, and a member of the Dalton Cardiovascular Research Center and Institute for Data Science and Informatics at the University of Missouri.
Source-Medindia
 MEDINDIA
MEDINDIA
 Email
Email