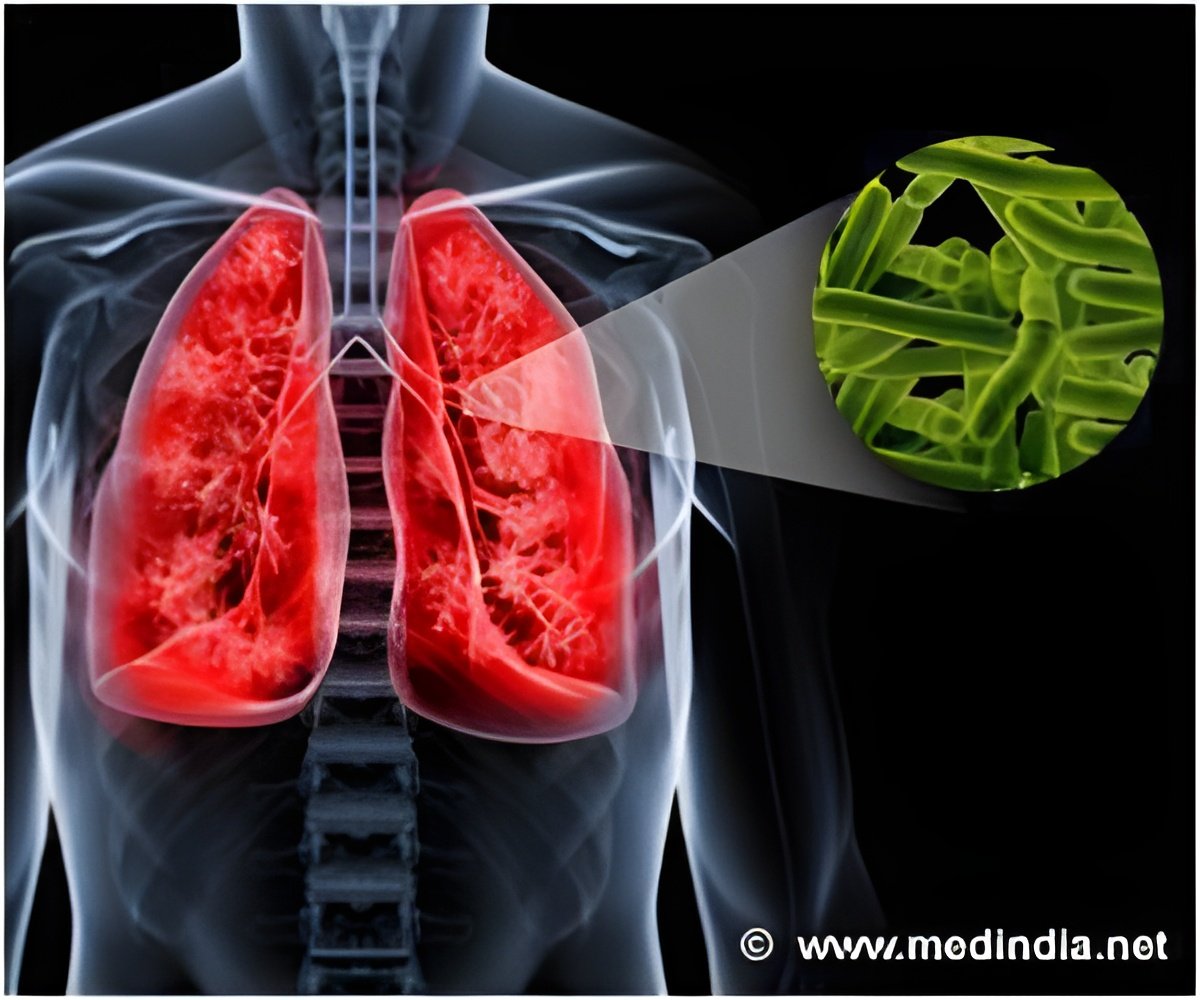
In 2017, 10 million people suffered and 1.6 million died of tuberculosis. New study explored the underlying mechanism of how the tuberculous bacterium is escaping from immune cell attack.
TOP INSIGHT
Targeting the production of 1-tuberculosinyladenosine could kill tuberculosis bacteria inside the macrophage. Also, targeting the enzyme Rv3378c which is specific to the bacteria could be the promising approach to treat tuberculosis.
Read More..
Virulence
In a previous study published in 2014, the team identified lipids present in M. tuberculosis but not in M. bovis, a bacterium which is much less virulent. The lipids specific to M. tuberculosis could play a role in virulence. Indeed, an important candidate was found and identified as 1-tuberculosinyladenosine (1-TbAd), an adenosine modified by the attachment of a lipid in the 1-position. ’Such a modification is extremely rare in nature’, says Buter, ’yet M. tuberculosis produces and releases a relatively large amount of this compound’.
Two enzymes critical to the production of 1-TbAd were identified, but the mechanism by which this molecule helped the tuberculosis bacterium to survive remained a mystery. ’Then, we found research performed by another group in 2004, in which it was shown that the fusion of the phagosome and lysosome was blocked due to these enzymes. As the phagosome needs to be acidic for fusion, this led us to the hypothesis that 1-TbAd played a role in preventing acidification of the phagosome’.
Antacid
The group performed a series of experiments to rule out the possibility that the adenosine compound acted through a receptor. The findings confirmed that 1-TbAd does indeed work by reducing the acidity directly. Buter synthesized different variants of the molecule to determine which parts of the molecule were vital for its function. ’The lipid part is needed to cross the membranes and get into the phagosomes and lysosomes’, says Buter.
Microscopy studies performed in the lab of Nicole van der Wel at the Amsterdam UMC reveal that exposing macrophages to 1-TbAd makes their lysosomes swell up to five times their normal size. Tests with macrophages infected with M. tuberculosis show that the phagosomes only swell significantly in the presence of the enzyme Rv3378c needed to produce 1-TbAd. ’There are several mechanisms preventing the macrophages from killing M. tuberculosis, but we discovered what appears to be a key mechanism for bacterial survival’.
Interestingly, the mechanism by which 1-TbAd works is the same as how chloroquine kills the malaria parasite. ’This drug blocks the functioning of parasite’s lysosomes’. It suggests that 1-TbAd could be used as an anti-malarial drug. ’But also, targeting the production of 1-TbAd could kill M. tuberculosis inside the macrophages. The enzyme Rv3378c would be an interesting target for drug discovery, as the enzyme is unique to the tuberculosis bacterium’.
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email