Micra Transcatheter Pacing System is small enough to be delivered through a catheter and implanted directly into the heart to treat irregular heart beat.
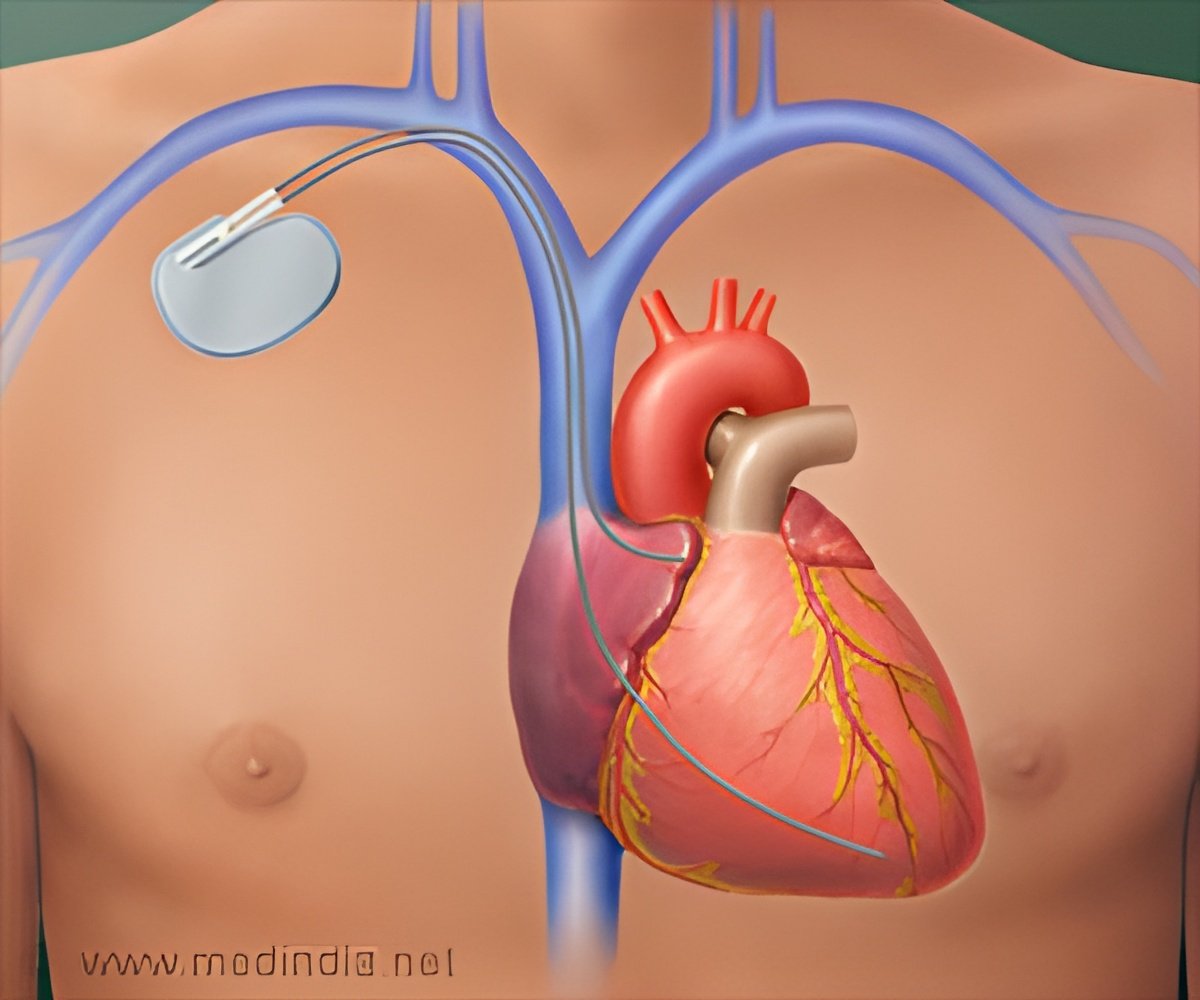
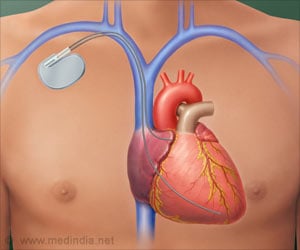
- The Micra Transcatheter Pacing System (TPS), recently approved by the US Food and Drug Administration (FDA), is a newly designed pacemaker. //
- The pacemaker is one tenth the size of the traditional device and provides patients with the most advanced pacing technology.
- TPS is small enough to be delivered through a catheter and implanted directly into the heart, providing a safe alternative to conventional pacemakers.
TOP INSIGHT
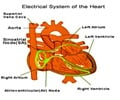
Pacemakers are the most common way to treat bradycardia to help restore the heart's normal rhythm and relieve the symptoms by sending electrical impulses to the heart to increase the heart rate.
Unlike traditional pacemakers, this one does not require cardiac wires (leads) or a surgical "pocket" under the skin to deliver a pacing therapy.
When the heart rate is low, it renders the heart incapable of pumping enough oxygen-rich blood to the body during normal activity or exercise, thereby causing dizziness, fatigue, shortness of breath or fainting spells.
"The device is small enough to be delivered through a catheter and implanted directly into the heart, providing a safe alternative to conventional pacemakers without the complications associated with leads," said Schurmann.
"The device also allows us to automatically adjust pacing therapy based on a patient's activity levels and another positive is the battery can last up to 10 years," he said.
"This is not a complicated procedure and the first patient that we implanted is doing extremely well," said Paul Schurmann, from Houston Methodist Hospital in the US.
Source-Medindia
 MEDINDIA
MEDINDIA

 Email
Email