Pfizer's RSV injection lowered the risk of RSV infection in older persons in trials. The FDA advisory group will also review a comparable shot from GSK.
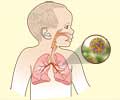
- RSV is the last of the major respiratory viruses that affect our population each year
- It normally causes mild cold-like symptoms in adults, although lung infections or pneumonia are possible. RSV can aggravate critical illnesses such as asthma, chronic obstructive lung disease, and congestive heart failure
- The US FDA is expected to decide on RSV vaccination approval for adults aged 60 and above
First RSV Vaccination Approved by US FDA
The FDA must then approve the vaccine, which might take several months, even though the agency normally follows the recommendations of the advisory group. Before the shot is made available to the public, the Centers for Disease Control and Prevention must endorse it after FDA clearance. If all of this occurs, it will be the first RSV vaccine to be licensed in the United States.RSV Vaccination developed by GSK
The second could be right on its tail. The advisory council will reconvene to assess the safety and efficacy of a similar RSV vaccination for older individuals, this one developed by GSK. The shot reduced the chance of symptomatic sickness by 83% and severe disease by 94% in persons aged 60 and up, according to trial findings published in the New England Journal of Medicine.What is RSV?
RSV causes lower respiratory disease, albeit most healthy adults experience very minor symptoms. RSV, on the other hand, can cause bronchiolitis, which inflames the airways and clogs them with mucus, or pneumonia in severe cases. Such results are especially dangerous for the elderly and babies. Every year, RSV kills around 10,000 persons aged 65 and above, as well as approximately 300 children under the age of five in the United States. Infections among infants increased substantially this winter, overwhelming children's hospitals - a reminder of the virus's threat.A separate vote analyzing the safety of the Pfizer vaccine was held at the advisory committee meeting, and the results were the same: seven votes in favor, four against, and one abstention.
Several of the experts who did not vote in favor based on the vaccine's efficacy voiced worry that there were insufficient RSV-infected trial participants to appropriately analyze the injection. Those who voted "no" based on the vaccine's safety profile were mostly concerned about Guillain-Barré syndrome, or GBS, a rare neurological illness that damages nerve cells and causes muscle weakness or paralysis.
“It was a 1 in 9,000 risks of GBS, which is concerning,” said Dr. Hana El Sahly, the FDA advisory committee chair, who voted against the shot based on its safety profile but in favor based on efficacy.
Pfizer has tested its RSV vaccination in pregnant women to see if the protection may be passed on to unborn children. According to the company's research, the vaccine lowered the risk of severe illness in newborns by 82% during the first 90 days of life and by 69% during the first six months.
But, the FDA committee's vote was confined to the vaccine's use in the elderly. Pfizer's evidence for pregnant women is still being reviewed by the FDA, with a decision likely in August.
Side-Effects of RSV Vaccine
The most prevalent side effects among older persons, according to an FDA briefing sheet provided before the hearing, were weariness, headache, discomfort at the injection site, and muscle pain. The text also mentioned the possibility of Guillain-Barré syndrome.After receiving the vaccination, one male in Pfizer's experiment acquired Guillain-Barré syndrome, and another woman had Miller Fisher syndrome, a rare nerve condition similar to Guillain-Barré.
The most common side effects of GlaxoSmithKline's vaccination were identical to those of Pfizer's — injection site pain, weariness, and muscular soreness — although participants in that trial reported side effects more frequently than those in Pfizer's.
Many businesses have been rushing to win FDA approval for RSV vaccinations. According to PATH, a nonprofit global health organization, eleven RSV vaccinations are now being tested in clinical studies in the United States. According to Moderna, an RSV shot developed for older persons might be submitted to the FDA by July. By the middle of the year, Bavarian Nordic expects to obtain late-stage trial data for their RSV vaccine, which targets the same demographic.
In addition, the FDA is analyzing data from trials of a monoclonal antibody injection designed to protect babies from RSV, which works similarly to a vaccination. The injection, developed by Sanofi and AstraZeneca, is already approved in Europe.
Source-Medindia