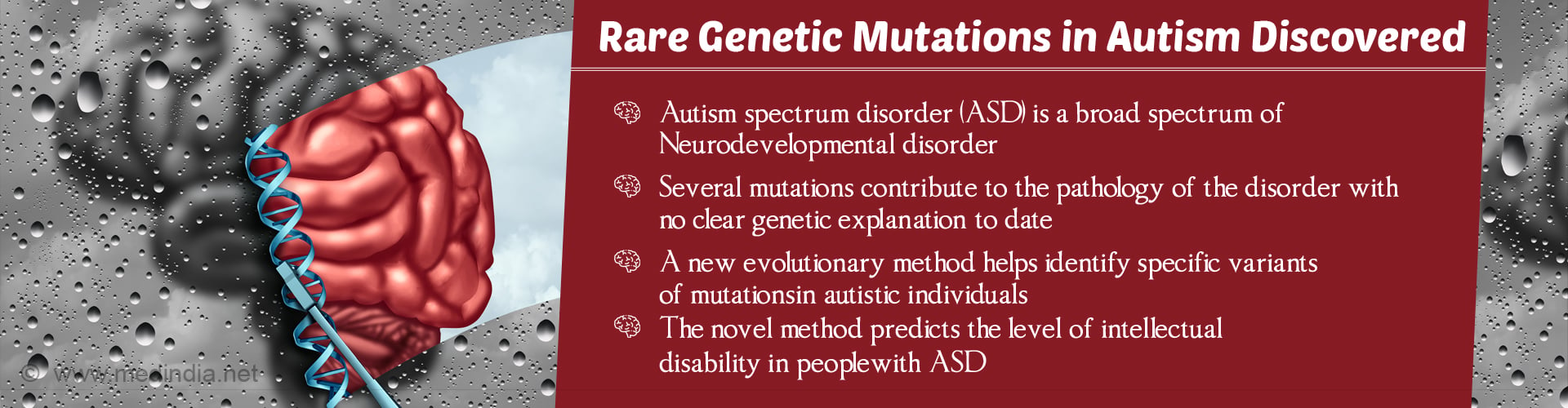
Novel computational model identifies the genes linked to autism that helps in predicting the level of intellectual disability in people with autism spectrum disorders (ASD).
- Recognizing specific mutations among hundreds of genes associated with autism spectrum disorders (ASD) has been challenging to determine till the present
- The novel computational method assists in distinguishing the genes that are most likely associated with autism spectrum disorders (ASD)
- Massive volumes of evolutionary data based on the analysis of de novo missense variants were fed into the model to segregate ASD patients and unaffected ones
- As a result, this would in turn help predict the severity of intellectual disability in ASD patients
New computational approach effectively identifies genes that are most likely associated with autism spectrum disorder (ASD). By applying only rare mutations in genes beyond those already associated with the syndrome, this method also helps in predicting the severity of intellectual disability in patients with ASD as per a study “A method to delineate de novo missense variants across pathways prioritizes genes linked to autism” at Baylor College of Medicine, published in the journal Science Translational Medicine.
TOP INSIGHT
A novel computational model identifies the genes linked to autism. This helps predict the level of intellectual disability in people with autism spectrum disorders (ASD) by utilizing only rare mutations in genes associated with the condition.
Read More..
Autism spectrum disorder (ASD) is a broad spectrum of disorders due to neurodevelopmental delay. It is primarily characterized by social, communication, and behavioral challenges.
The incidence of ASD has been increasing over the last two decades from 1 in 5,000 people in 1975 to 1 in 68 in 2014.
ASD has been associated with hundreds of genetic mutations (each mutation having a mild effect) that make it difficult to determine those mutations that are truly involved in the disease and those that are incidental.
Approximately only 2% of ASD cases are known to be affected by the most commonly mutated genes.
Recognizing exact genes that contribute to ASD might help in better understanding of the disease pathology and formulate more effective outcomes and treatments.
Finding the Specific ASD Mutation
Hence, the mutations that were most liable to be harmful were then focussed by further narrowing their uniqueness to each individual and how these mutations add up in each molecular pathway.
Missense Mutations in Autism
Mutations are generally defined as any change that occurs in the DNA sequence due to any error or influence of environmental factors. Certain mutations in ASD severely intrude the structure of proteins, thereby rendering them inactive, and are associated with the severity of ASD.
On the contrary, missense mutations are much more frequent and harder to assess than loss-of-function mutations. Missense mutations tweak the protein’s function a little or severely impair it, thereby making it difficult to interpret their impact.
It is reported that autistic people are more prone to carry a de novo missense mutation than a de novo loss-of-function mutation. De novo or new mutations are the types of mutations that seem to develop for the first time in a family member, with no history of inheritance from either parent.
Multi-layered Strategy to de novo Missense Mutations
The team enlisted a cohort of patients with ASD and their siblings as a whole to distinguish the causal group of genes and mutations among the varied de novo missense mutations by applying a multi-layered strategy.
The sequences of all the protein-coding genes in a group of de novo mutations were examined in 2,392 families with ASD that are in the Simons Simplex Collection.
Following that, the effect of each missense mutation on the fitness or functionality of the corresponding protein and its pathways was evaluated by utilizing a previously developed computational tool in the Lichtarge lab – the Evolutionary Action (EA) equation.
It was observed that among the 1,418 de novo missense mutations affecting 1,269 genes in the patient group, most genes were mutated only once.
“Knowing that ASD is a multigenic condition that presents on a spectrum, we reasoned that the mutations that were contributing to ASD could dispersed amongst the genes of a metabolic pathway when examined at a cohort level, rather than being clustered on a single gene. If any single component of a pathway becomes affected by a rare mutation, it could produce a clinical manifestation of ASD, with slightly different results depending on the specific mutation and the gene,” says first author Dr. Amanda Koire, a graduate student in the Dr. Olivier Lichtarge lab during the development of this project & a psychiatry research resident at Brigham and Women’s Hospital, Harvard Medical School.
Mutated Pathway Associated with ASD
The study shows that grouped de novo missense mutations implicated 398 genes from 23 pathways including axonogenesis – a pathway for the development of new axons in neurons in the brain, synaptic transmission, and other neurodevelopmental pathways.
The assemblage of all these data on different pathways could help identify the specific set of genes related to ASD.
"This opens doors on many fronts. It suggests new genes we can study in ASD, and that there is a path forward to advise parents of children with these mutations of the potential outcomes in their child and how to best involve external support in early development intervention, which has shown to make a huge difference in outcome as well," says co-author Young Won Kim, a graduate student in Baylor's Integrative Molecular and Biomedical Sciences Graduate Program working in the Lichtarge lab.
The authors speculate that this approach could be utilized to test a wide set of complex diseases via available genome sequence data. This would then aid in interpreting the rare mutations and better analysis of risk and morbidity associated with various genetic diseases.
Autism Fact Sheet
- 1 in 59 children are diagnosed with autism with more than 3.5 million Americans living with the disorder in the United States
- Globally 1 in 270 individuals are diagnosed with autism spectrum disorder, occurring in all racial and ethnic groups
- ASD is 4.3 times more common in boys (3.0%) than in girls (0.7%)
- Autism prevalence is inflated by 6-15% each year from 2002 to 2010
- Approximately 1 in 8 individual with autism has at least one neurodevelopmental condition and is rated to be about 10 times higher than the 1.3% reported in India’s 2011 census
- The cost of autism services in the U.S. approximates $236-262 billion annually
- Autistic people are often subject to social stigma and discrimination
- However the communication and social skills of an autistic individual can be improved with evidence-based psychosocial interventions, that may render a positive impact on their well-being
References:
- Autism Spectrum Disorders - https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders
- Autism - Facts and Statistics - https://www.autism-society.org/what-is/facts-and-statistics/
- Autism Spectrum Disorder - https://www.nimh.nih.gov/health/statistics/autism-spectrum-disorder-asd
- Evolutionary Action of de novo Missense Variants across Pathways Prioritizes Genes Linked to Autism and Predicts Patient Phenotypic Severity - https://www.biorxiv.org/content/10.1101/158329v1.abstract
Source-Medindia
 MEDINDIA
MEDINDIA



 Email
Email