Bone cancer Ewing sarcoma is driven by a genetic abnormality that operates through two distinct processes, activating genes that stimulate tumor growth and suppressing those that prevent cancer.
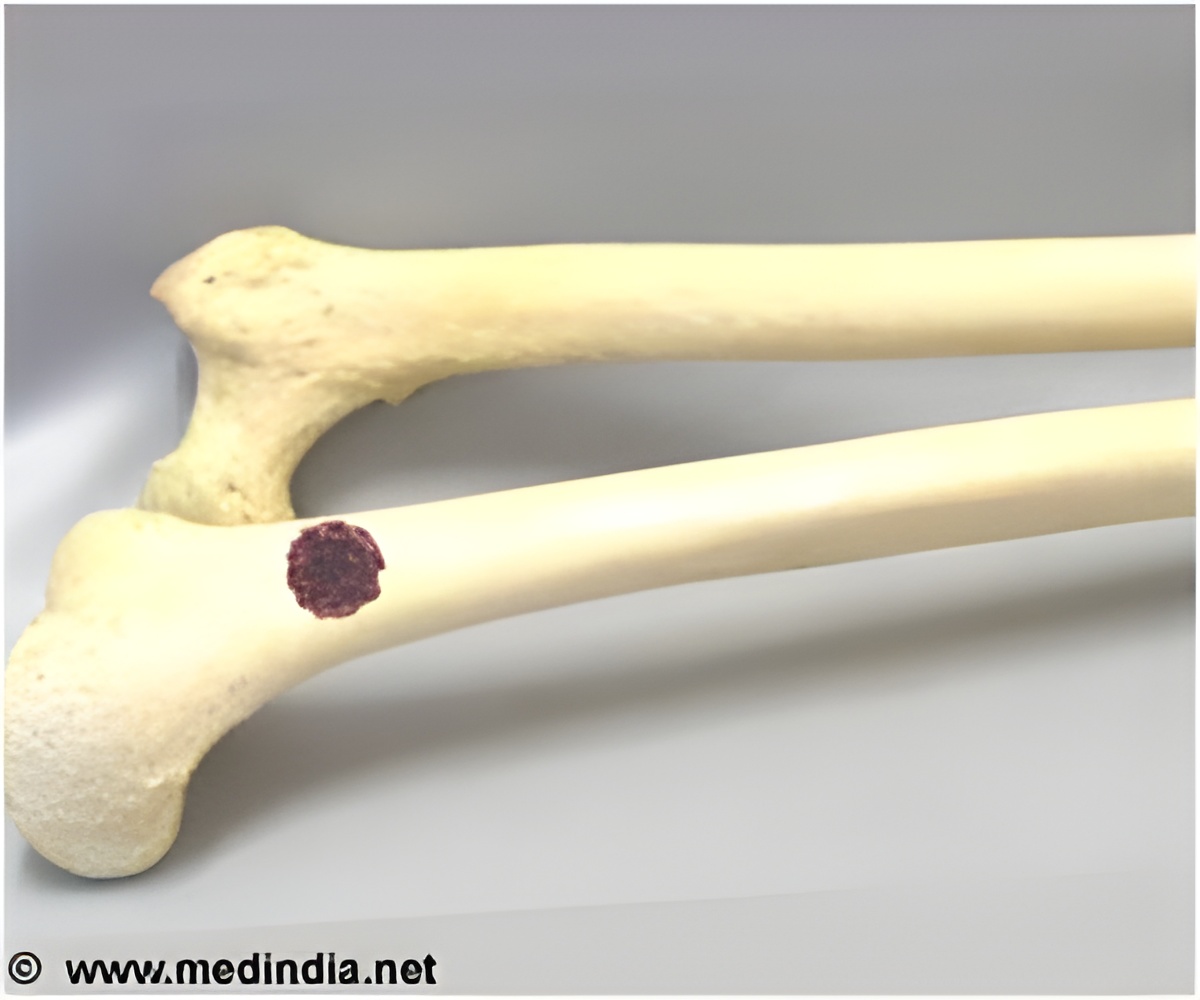
Led by Miguel Rivera, MD, and Bradley Bernstein, MD, PhD, of the MGH Department of Pathology and Center for Cancer Research, the investigators analyzed how regulatory changes resulting from the EWS-FLI1 fusion alter the activation and repression of regions across the genome. This approach, called chromatin profiling, is a powerful tool for determining how transcription factors act in cancer and revealed that EWS-FLI1 acts through two different mechanisms.
First the fusion protein converts common repetitive elements within the genome into active enhancing elements that stimulate the expression of other genes. This 'pioneer' function is remarkable, Rivera notes, because while these repeat segments have no known function outside the setting of Ewing sarcoma, they are critical for the development of the tumor. The second property of the EWS-FLI1 fusion is to turn off factors that regulate gene transcription at a different group of sites. In essence the abnormal protein both stimulates the abnormal cellular growth that leads to tumor formation and turns off factors that should keep that growth in check.
"Uncovering the molecular functions of EWS-FLI1 points to processes that may be targeted therapeutically" says Rivera, an assistant professor of Pathology at Harvard Medical School. "In addition, some of the genes directly turned on by EWS-FLI1 may themselves be therapeutic targets. One such gene, the kinase VRK1, is regulated by an EWS-FLI1-dependent enhancer that is active in Ewing sarcoma cell lines, and we found that its expression is essential for the survival of Ewing sarcoma cells. These studies also underscore the importance of performing epigenetic analysis to understand the biological pathways that are altered in cancer."
Source-Eurekalert
 MEDINDIA
MEDINDIA


 Email
Email