- Enhanced diagnostic accuracy with serological biomarkers and AI models
- FDA approval of Resmetirom for non-cirrhotic NASH marks a milestone
- Lifestyle interventions like Mediterranean diet and intermittent fasting show promise
FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease
Go to source). Recent advancements in diagnostic modalities, including serological biomarkers and AI-driven imaging techniques, offer promising non-invasive approaches to assess disease severity accurately. Concurrently, the emergence of novel therapeutic agents, such as Resmetirom and Aldafermin, signals a pivotal shift in the management paradigm, offering targeted interventions to halt the disease progression and improve patient outcomes.
AI-driven diagnostics and novel therapeutics are reshaping NASH care, offering hope beyond biopsies. #nash #liver #medindia’
NASH Diagnostics
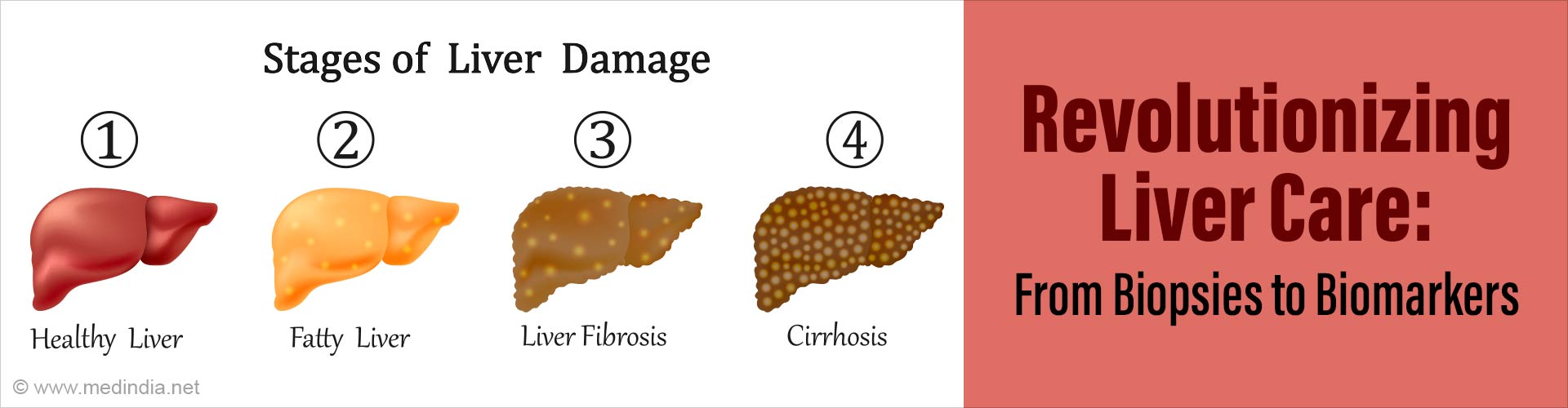
Non-alcoholic fatty liver disease (NAFLD) is a prevalent condition characterized by excessive fat accumulation in the liver. It's more severe form, non-alcoholic steatohepatitis (NASH), can lead to liver fibrosis, cirrhosis, and hepatocellular carcinoma. Effective diagnostics are essential for early detection and management. Recent advancements in diagnostic techniques have shown promise in accurately assessing severity of liver fibrosis severity and diagnosing NAFLD in a non-invasive way.1. Serological Biomarkers vs. Liver Biopsies:
Several serological biomarkers have been compared with liver biopsies to assess their diagnostic accuracy in predicting liver fibrosis severity in individuals with metabolic associated fatty liver disease (MAFLD). Notably, the FIB-4 index for any fibrosis, FibroMeter for significant fibrosis, ELF (Enhanced Liver Fibrosis) for advanced fibrosis, and FIB-4 for cirrhosis have shown superior diagnostic accuracy compared to traditional liver biopsies.
2. Novel Tests for NAFLD Diagnosis:
Studies have identified a series of tests, including 20-carboxy arachidonic acid (20-COOH AA) and 13,14-dihydro-15-keto prostaglandin D2 (dhk PGD2), as potentially the most accurate non-invasive tests for diagnosing NAFLD. These tests offer a promising alternative to invasive procedures like liver biopsy.
3. AI-Based Diagnostic Models:
Artificial intelligence (AI) models leveraging ultrasound (US) imaging and clinical data have demonstrated reliability in detecting NAFLD and its complications. These models not only enhance diagnostic accuracy but also help reduce costs associated with invasive procedures like liver biopsy, making them a valuable tool in clinical practice.
NASH Therapeutics
Effective therapeutics are crucial for managing NAFLD and NASH, aiming to halt disease progression and improve patient outcomes. Recent developments in NASH therapeutics have introduced promising treatment options targeting liver fibrosis and resolution of NASH.1. Resmetirom (NDA: 217785) Approval:
Resmetirom, a thyroid hormone receptor-beta (THR-beta) agonist, has received approval from the US Food and Drug Administration (FDA) for the treatment of non-cirrhotic NASH with moderate to advanced liver fibrosis (stages F2 to F3 fibrosis). This approval marks a significant milestone in NASH therapeutics, providing clinicians with a novel treatment option to address the unmet medical needs of patients with NASH.
2. Aldafermin for NASH Cirrhosis:
Clinical trials have demonstrated the efficacy of Aldafermin, with a significant reduction in Enhanced Liver Fibrosis observed in patients with compensated NASH cirrhosis. This highlights the potential of Aldafermin as a therapeutic agent for advanced stages of NASH.
3. Efficacy of Resmetirom:
Studies have shown that both the 80-mg and 100-mg doses of Resmetirom are superior to placebo in achieving NASH resolution and improving liver fibrosis by at least one stage. These findings underscore the therapeutic potential of Resmetirom in treating NASH and addressing progression of liver fibrosis progression.
4. Dietary Interventions for NAFLD:
Mild weight loss induced by a Mediterranean-like diet adapted for Asians has demonstrated multiple beneficial health effects in females with NAFLD. Additionally, a combination of the Mediterranean diet with intermittent fasting has shown long-term benefits in improving steatosis in NAFLD patients compared to the Mediterranean diet alone. Both early and late time-restricted eating have led to significant reductions in intrahepatic fat and improvements in metabolic health among NAFLD patients.
In conclusion, recent advancements in NASH diagnostics and therapeutics offer promising opportunities for improved patient care and outcomes. Non-invasive diagnostic techniques, AI-based models, and novel therapeutic agents provide clinicians with valuable tools for early detection, accurate assessment, and effective management of NAFLD and NASH.
Resmetirom approved by US-FDA for the treatment of non-cirrhotic non-alcoholic steatohepatitis (NASH) with moderate to advanced liver fibrosis (consistent with stages F2 to F3 fibrosis). Annual cost of therapy $47400 : approximately INR40lacs which means INR11K/day.
Additionally, lifestyle interventions such as dietary modifications and intermittent fasting present complementary approaches to enhance treatment efficacy and promote liver health in individuals with NAFLD.
Reference:
- FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease - (https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease)
Source-Medindia