Tildrakizumab-asmn Medication Information
Get detailed information on Tildrakizumab-asmn, including pronunciation, uses, dosage guidelines, indications, and instructions on how and when to take it and when to avoid it.
The updated prescription information on Tildrakizumab-asmn provides an overview of possible side effects, precautions, warnings, and storage tips.
You'll also find brand names used in india and internationally, along with pricing details. For further clarification, consult your healthcare provider.
Generic Name : Tildrakizumab-asmn Pronunciation : til-dra-KIZ-eu-mab-asmn Therapeutic Classification : Monoclonal antibodiesTrade Names/Brand Names of Tildrakizumab-asmn
India :
Ilumya
Overview of Tildrakizumab-asmn
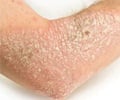
• Tildrakizumab-asmn injection was approved by FDA to treat adults with immunologically mediated inflammatory disorders such as moderate to severe plaque psoriasis.Why is Tildrakizumab-asmn Prescribed? (Indications)
Tildrakizumab-asmn is an interleukin-23 antagonist which is prescribed for treating adults suffering from moderate to severe plaque psoriasis who may benefit with systemic therapy or phototherapy.Tildrakizumab-asmn is a monoclonal antibody which works by binding to interleukin-23 and blocking it from attaching to its receptor; it thus reduces the release of cytokines and chemokines which are responsible for causing inflammation.
When should Tildrakizumab-asmn not be taken? (Contraindications)
Tildrakizumab-asmn should not be used in patients• Allergic to tildrakizumab-asmn
• Pregnant and breastfeeding
• With any active infection (e.g., tuberculosis)
• With a low immune system
• Under 18 years of age
What is the dosage of Tildrakizumab-asmn?
• The recommended adult dose is 100 mg to be given at week 0 and at week 4 followed by a dose every 12 weeks after that.How should Tildrakizumab-asmn be taken?
• Tildrakizumab-asmn is available as an injection or a pre-filled syringe which should be administered by a healthcare provider subcutaneously (just under the skin).• Pre-filled syringes should be used only once and discarded after that.
What are the warnings and precautions for Tildrakizumab-asmn?
• Patients should be evaluated for the presence of tuberculosis (TB) infection before starting treatment with tildrakizumab-asmn. If the patient is suspected of TB infection, the treatment should not be initiated.• Patients should be advised to consult the physician if any signs or symptoms of acute or chronic infections are observed, as tildrakizumab-asmn can increase the severity of the infections. Discontinue tildrakizumab-asmn therapy until the infection resolves.
• Patients can develop neutralizing antibodies to tildrakizumab while taking tildrakizumab-asmn as a long-term therapy resulting in decreased serum concentration of tildrakizumab-asmn or treatment failure.
What are the side effects of Tildrakizumab-asmn?
• Diarrhea• Upper respiratory tract infections
• Nasopharyngitis
• Urticaria
• Pruritus
• Pain, swelling, redness or bruising at the injection site
What are the other precautions for Tildrakizumab-asmn?
• Before administration, place the tildrakizumab-asmn injection out of the refrigerator for 30 minutes to come to room temperature.• Check the injection for the presence of any particulate matter or discoloration; if so discard the medication and use a fresh one.
• The injection sites are thighs, abdomen, or upper arm. Never inject inside the 2-inch circumference around the navel.
• Do not inject onto scars, stretch mark areas, psoriasis affected sites, tender or bruised skin.
What are the Drug Interactions of Tildrakizumab-asmn?
• The concentration of dextromethorphan was found to increase when administered together with tildrakizumab-asmn 200 mg and therefore should be avoided.• Do not take live vaccines such as MMR, Herpes zoster, or yellow fever vaccine while on treatment with tildrakizumab-asmn as it results in severe infection.
What are the storage conditions for Tildrakizumab-asmn?
• Tildrakizumab-asmn injection should be stored in the refrigerator at 2°C to 8°C.• Should be kept in the original carton and protected from light.
• Do not shake or freeze.
• Tildrakizumab-asmn can be stored at room temperature (below 25°C) for a maximum of 30 days but should be discarded after 30 days if not used.
• Do not place tildrakizumab-asmn injection in the refrigerator if it has already been kept at room temperature.