Novel technology, called GelCORE can treat corneal injuries without the need for surgery, finds a new study.

‘Corneal injuries are common causes of vision impairment globally. But, a new technology, called GelCORE (gel for corneal regeneration) can seal the wounds on the cornea and help in regenerating the corneal tissue. This biomaterial could one day decrease the need for surgery.
’
Read More..




"We set out to create a material that is clear, strongly adhesive and permits the cornea to not only close the defect but also to regenerate. We wanted this material to allow the cells of the cornea to mesh with the adhesive and to regenerate over time to mimic something as close to the native cornea as possible."Read More..
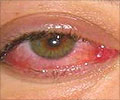
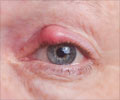
Corneal injuries are a common cause of visual impairment worldwide, with more than 1.5 million new cases of corneal blindness reported every year. The current standard of care for filling in cuts, thinning areas, or holes in the cornea (corneal defects) includes the use of synthetic glues or surgery to patch the eye with a tissue and corneal transplants.
The synthetic glues currently available are rough, inherently toxic to tissues, difficult to handle, and can lead to significant vision loss due to the material's opacity and poor integration with corneal tissues. Corneal transplants carry risks of post-transplant complications, including infection or rejection.
With the goal of addressing this unmet clinical need, researchers on the Science Advances report set out to develop an adhesive designed for long-term integration with the cornea.
The team engineered an adhesive biomaterial, GelCORE, made of chemically modified gelatin and photoinitiators, which are activated by a short-time exposure to blue light. Initially, the gel is a clear, viscous material designed to be applied with a dropper or syringe. When exposed to light, the material hardens, taking on the biomechanical features of a native cornea. And, over time, the cornea cells gradually grow into and become one with this material. Thus, GelCORE is similar to the native cornea highly transparent, able to bond to the native tissue, and capable of supporting cell and tissue regeneration.
Advertisement
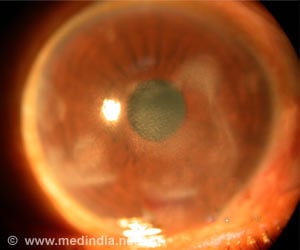
In the Science Advances report, the researchers describe their assessments of GelCORE in a preclinical model of corneal injury. They applied GelCORE at 20 percent concentration to corneal defects of 3mm, and then applied visible light for 4 minutes. Immediately after the light exposure, they observed firm adhesion of the gel to the corneal defect. One day later, they observed a transparent, smooth eye surface, with a surrounding cornea that was clear and without inflammation. One week after application, the gel could still be observed on the defect site in the cornea and remained transparent. Over time, the tissue showed signs of regeneration, with cells of the new tissue showing similarities between regenerated tissue and native tissue.
Advertisement
"We envision, if a patient comes in with a big laceration, they might receive formulation A. If they come in with a corneal scar, they might get formulation B." The authors also hope to begin clinical trials to test the technology in human patients in approximately one year.
Source-Eurekalert