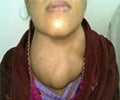
Researchers at the Mayo Clinic say the drug pazopanib may help revolutionize the care of patients with metastatic, rapidly progressive differentiated thyroid cancers.
Researchers at the Mayo Clinic say the drug pazopanib may help revolutionize the care of patients with metastatic, rapidly progressive differentiated thyroid cancers. They are publishing findings of a phase II clinical trial in The Lancet Oncology.
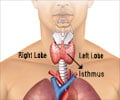
The researchers studied 37 patients with the most aggressive form of this cancer -- developing in less than 5 percent of patients with differentiated thyroid cancer -- and found that about half (18) patients had a long-lasting response to pazopanib. Of that group, 12 are still alive without disease progression. The median progression-free survival time was 11.7 months, with an overall survival rate of 81 percent at one year.The researchers say that, to their knowledge, these findings represent by far the highest response rate yet reported in such aggressive cases of differentiated thyroid cancer. They caution however, that this drug is not meant to be used in slow-growing differentiated thyroid cancers and that they cannot assess the survival advantage pazopanib offers to the patients studied.
Determining survival benefit would require a randomized clinical trial testing the agent, which inhibits all three vascular endothelial growth factor (VEGF) receptors, compared to other treatments or a placebo.
"In this group of patients, we would have expected the cancer to have progressed in everyone within six months, but instead the median time to progression was almost a year in response to pazopanib therapy," says Keith Bible, M.D., Ph.D., a medical oncologist and researcher who led the multicenter clinical trial, funded by the National Cancer Institute. Most of the patients treated were enrolled at Mayo Clinic campuses in Minnesota and Florida.
But as encouraging as this response is, it does not come without the potential for significant side effects, Dr. Bible says. The drug dose used in 16 patients had to be lowered because side effects were judged by oncologists to become potentially threatening or debilitating, and two patients experienced significant bleeding. Further, although two patients died in association with pre-existing disease while enrolled in the study, the agent could have contributed in some way, he says.
"Further studies of pazopanib in advanced thyroid cancer remain ongoing at Mayo Clinic and associated cancer centers to continue to learn more about how best to use the drug in these cancers," Dr. Bible says. "Such clinical trials also may provide patients access to this drug, which otherwise may be unobtainable due to cost, given that it is not yet approved for use in thyroid cancers."
The authors declare no conflicts of interest. The manufacturer of pazopanib (GlaxoSmithKline) did not provide funding or other material support to the researchers and did not have access to the data.
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email