Adverse events that had been noted in a pivotal clinical trial in women age 60 to 90 years old showed no tendency to increase after a further 3 years of treatment.
TOP INSIGHT
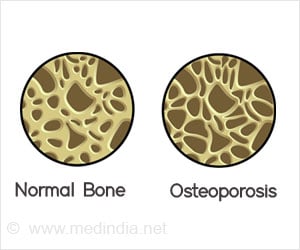
Adverse events that had been noted in a pivotal clinical trial in women age 60 to 90 years old treated for 3 years showed no tendency to increase after a further 3 years of treatment.
"All of this is consistent with an excellent safety and tolerability profile for denosumab treatment for osteoporosis," said Dr. Nelson Watts, lead author of the study results published in Journal of Bone and Mineral Research. The authors noted that, especially in older women on long-term treatment, many if not all adverse events could be called "life events" -- things that would have happened whether or not the person was participating in a clinical trial.
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email