‘The purpose of the Phase III study with AKP02 is to demonstrate good therapeutic efficacy for the treatment of mild to moderate psoriasis on both the body and scalp.’
Read More..Tweet it Now
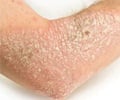
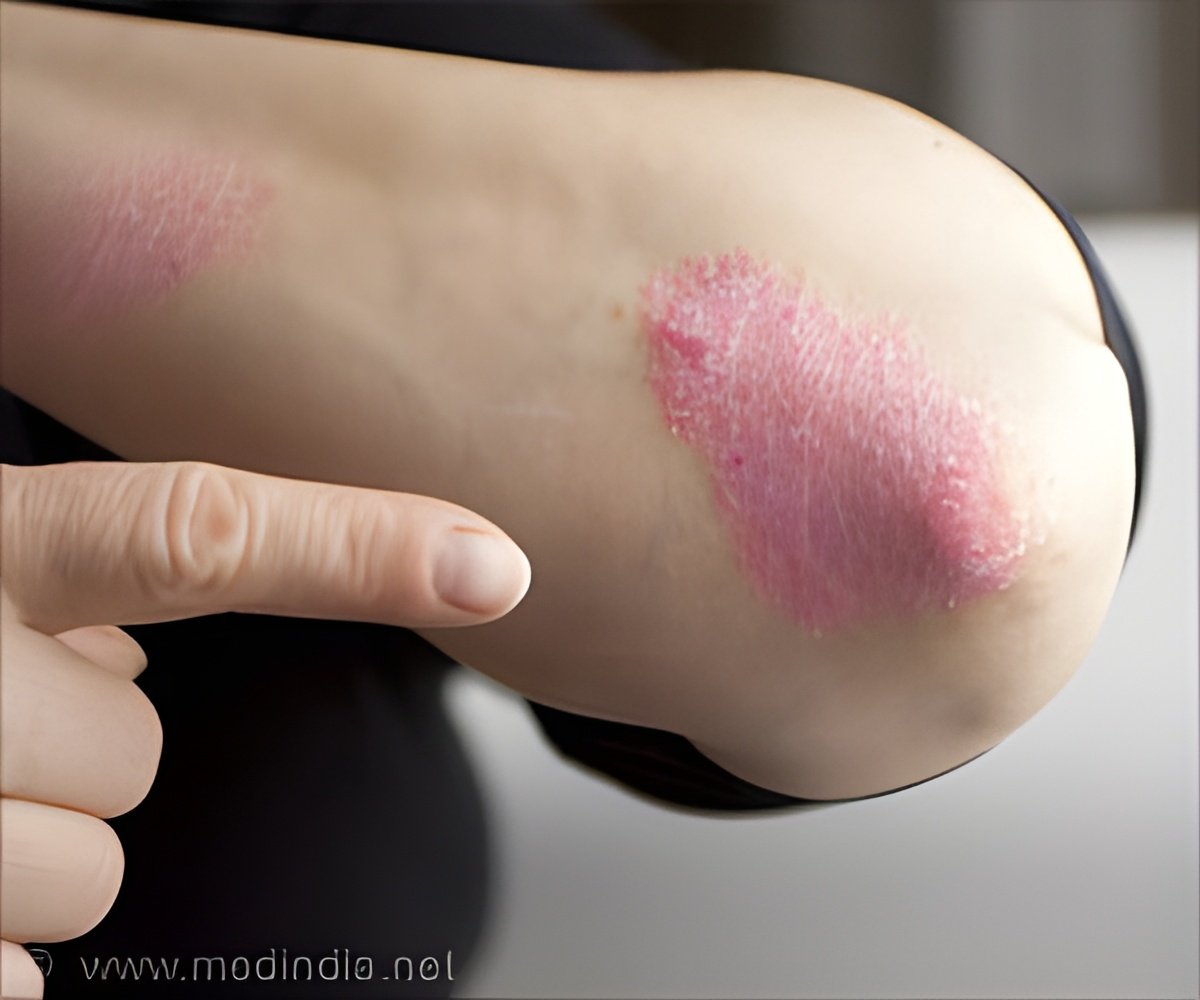
The aim with AKP02 is to offer a patient-friendly, spray-based treatment for mild to moderate psoriasis.
Read More..
Drug Lipidor and the Studies Based on the Drug
The drug candidate AKP02 combines calcipotriol and betamethasone and is based on Lipidor’s patented AKVANO® technology. Positive results from the Phase III study with the drug candidate AKP01, based solely on calcipotriol, show that Lipidor’s AKVANO® technology works well for the treatment of psoriasis.The preclinical studies show that betamethasone works well with AKVANO® — a positive requirement for the second drug candidate, which is a combination of both calcipotriol and betamethasone.
The purpose of the Phase III study with AKP02 is to demonstrate good therapeutic efficacy for the treatment of mild to moderate psoriasis on both the body and scalp.
Lipidor has chosen to compare the sprayable drug candidate AKP02 with Enstilar which is a commonly prescribed preparation in foam form for topical treatment of this indication and which contains the same combination of active substances as AKP02.
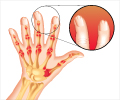
To reach a larger patient population, the scalp has also been included in the study. Psoriasis on the scalp, which often is affected, can consist of anything from weak to severe scale formation and cause varying degrees of itching.
Advertisement
Lipidor’s partner Aurena Laboratories will be responsible for the commercial production of the product.
Advertisement
"We are pleased to have recently signed an exclusive license agreement with RELIFE, a company in the Menarini Group which has a strong European and global presence. Altogether, this strengthens our quest to make these innovative new drug candidates AKP01 and AKP02 available to psoriasis patients in the hope that they may improve the treatment of their condition and their quality of life."
Lipidor is closely monitoring developments and taking measures to minimize or eliminate the impact on the company’s operations.
Source-Medindia