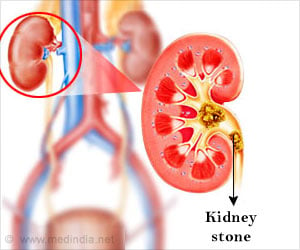
The company has revamped an internal mechanism inside the reusable device that had been almost impossible to disinfect before being used in the next patient.

‘The U.S. Food and Drug Administration has cleared Japan's Olympus Corp's duodenoscope with changes to the device's design and labeling intended to help reduce the risk of bacterial infections.’





A U.S. Senate report concluded that 25 outbreaks, including two in Los Angeles in which three people died were linked to dirty scopes made by Olympus.The company has revamped an internal mechanism inside the reusable device that had been almost impossible to disinfect before being used in the next patient.
Many of the illnesses were from a superbug known as CRE, or carbapenem-resistant Enterobacteriaceae, which can kill as many as half its victims.
The new revamped duodenoscope has been approved by the U.S. Food and Drug Administration and the company will begin collecting the scopes from hospitals next month to replace it with the new device.
“The company had modified the design to create a tighter seal and reduce the risk that patient fluids and tissue could leak into the closed channel. Olympus executives estimate the firm can replace the mechanism in all 4,400 devices in use in the U.S. by August. Until then, hospitals can continue to use the scope — known as the TJF-Q180V — but should “meticulously follow” the company's cleaning instructions,” said the FDA.
Advertisement
“The steps taken today are important, but there is much more we need to do to make sure the FDA can respond quickly and appropriately when problems with medical devices occur,” Murray said.
Advertisement