Mandibular advancement devices can help against daytime sleepiness in patients with obstructive sleep apnoea. Treatment with a MAD is not inferior to positive airway pressure (PAP) therapy with a sleep mask, reports a new study.
TOP INSIGHT
People with obstructive sleep apnoea have difficulties breathing while sleeping, including breathing pauses, and are very sleepy during the day.
Read More..
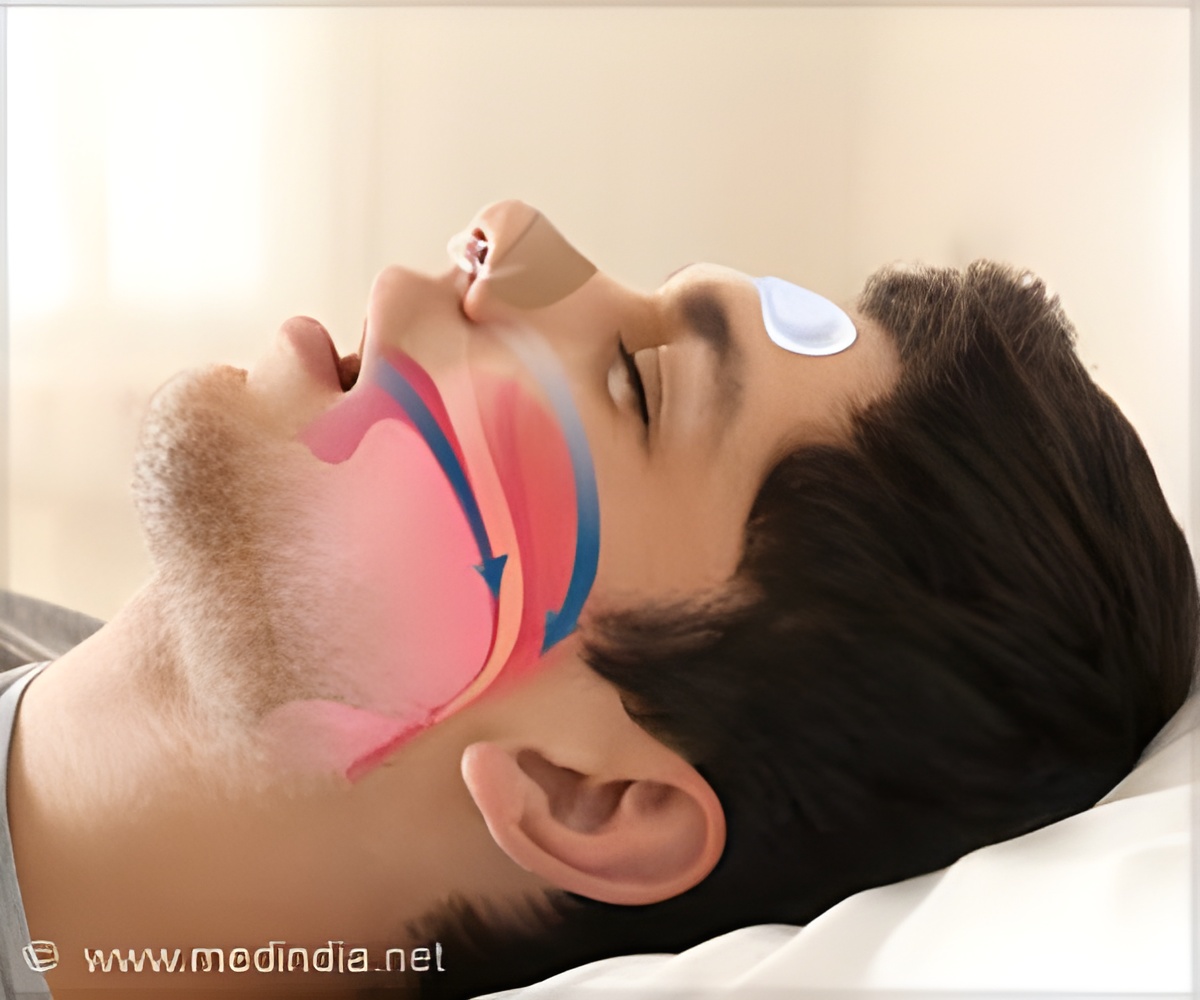
A mandibular advancement device keeps the airways open mechanically
The treatment of obstructive sleep apnoea depends on the severity of the disease. If it is mild, conservative measures such as weight reduction, sleep hygiene measures (no alcohol, no smoking), or positional therapy (avoiding lying on the back while sleeping) can alleviate the symptoms. For a higher degree of severity, PAP therapy with a sleep mask is used as standard treatment, where ventilation with positive pressure is used to keep the patients' airways open.
According to the relevant clinical practice guideline, mild to moderate obstructive sleep apnoea can also be treated with a MAD. The plastic splint in the mouth, fitted by a dentist or orthodontist, then ensures that the lower jaw is held further forward, keeping the upper airway mechanically open. This method is usually well-tolerated and is, in many cases, preferred by patients over PAP therapy. However, MAD treatment is not suitable for all patients: In cases of toothlessness, insufficient remaining teeth or pronounced periodontitis, for example, the plastic splint cannot be worn.
Treatment with a MAD is not inferior to PAP therapy
In comparison to no treatment or treatment with a placebo splint (research question 1), IQWiG researchers infer an indication of the benefit of wearing a MAD for the patient-relevant outcome "daytime sleepiness." This benefit is not challenged by the results on other patient-relevant outcomes (including sleep quality, cognitive performance, depressive symptoms, and headaches). No data were available for the outcomes of overall mortality or survival and cardiovascular morbidity. In comparison with PAP therapy (research question 2), the IQWiG researchers infer an indication of the non-inferiority of MAD versus PAP therapy for the patient-relevant outcome "daytime sleepiness." With regard to other patient-relevant outcomes, there was no disadvantage of MAD compared with PAP therapy either. No usable data were available for the outcomes of overall mortality or survival and cardiovascular morbidity.
After the hearing: "hint" becomes "indication."
In comparison with the preliminary report, the IQWiG project team upgraded the conclusions on the benefit of MAD from "hint" to "indication," both in comparison with no treatment or placebo (research question 1) and for the non-inferiority of MAD versus PAP therapy (research question 2). This also happened because, in the context of the hearing on the preliminary report, the participants submitting comments pointed out calculation errors in the probability calculations, which IQWiG has now corrected. Among other things, this resulted in different correlation coefficients for the assessments. In addition, a new study also provided additional evidence for research question 2 (MAD vs. PAP therapy). This led to more robust assessment results for both research questions regarding the outcome "daytime sleepiness."
Source-Eurekalert
 MEDINDIA
MEDINDIA



 Email
Email