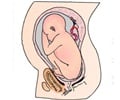
Pregnancy is not recommended in patients with certain types of heart disease - for example, pulmonary arterial hypertension, severely dilated aorta, or severely reduced ability of the heart to pump blood.

TOP INSIGHT
Beyond 40 weeks, pregnancy has no added benefit for the baby and may even have negative effects.
Heart disease in pregnancy is increasing as more women with congenital heart disease reach adulthood due to improved treatment and as the age at first pregnancy rises, accompanied by the higher rates of ischaemic heart disease in older, compared to younger, women. Cardiovascular risk factors including hypertension, diabetes and overweight are also on the rise in pregnancy as older women become pregnant and women now acquire risk factors at a younger age.
The guidelines provide recommendations on in vitro fertilisation (IVF), contraception, and termination of pregnancy in women with heart disease. IVF often uses high doses of hormones, which increase the risk of thrombosis and heart failure, so women with heart disease need a cardiologist's confirmation that the chosen method is safe. Since carrying more than one baby puts more stress on the heart, women with heart disease undergoing IVF are strongly advised to transfer a single embryo. Girls with congenital heart disease need contraception advice to avoid unplanned pregnancy. Some contraception methods are contraindicated in patients with certain types of heart disease.
For drugs used to treat heart disease, the guidelines list information on adverse events obtained from human and animal studies. In addition, the guidelines state: "In the case of an emergency, drugs that are not recommended by the pharmaceutical industry during pregnancy and breastfeeding should not be withheld from the mother. The potential risk of a drug and the possible benefit of the therapy must be weighed against each other."
Professor Vera Regitz-Zagrosek, Chairperson of the Guidelines Task Force and Director of the Institute for Gender Medicine, Charité University Medical Centre Berlin, Germany, said: "When drug companies have no data on whether a drug is safe during pregnancy and breastfeeding they tend to say it is not recommended. It may be appropriate to give a drug to a severely ill woman if there are no harmful side effects noted in the databases listed in the guidelines."
Professor Roos-Hesselink said: "The delivery plan should be available 24 hours a day so that when a pregnant woman with heart disease arrives at hospital in labour hospital staff know exactly what to do."
Source-Eurekalert
 MEDINDIA
MEDINDIA



 Email
Email