India launches its first indigenous CRISPR gene therapy to transform Sickle Cell Disease care and expand access for underserved tribal communities.
- India unveils its first indigenous CRISPR based gene therapy for Sickle Cell Disease
- BIRSA 101 aims to make advanced treatment accessible for underserved tribal populations
- The breakthrough strengthens India's role in delivering affordable cutting edge healthcare
Union Minister Dr. Jitendra Singh launches India's first indigenous "CRISPR" based gene therapy for Sickle Cell Disease, which particularly affects India's tribal population
Go to source). The therapy, titled “BIRSA 101,” honors Bahagwan Birsa Munda on the occasion of his 150th birth anniversary and recognizes his legacy as a prominent tribal freedom fighter.
During the announcement, Union Minister of State for Science and Technology; Earth Sciences; MoS PMO, Personnel, Public Grievances, Pensions, Atomic Energy and Space, Dr. Jitendra Singh stated that the country has officially embarked on a decisive mission to eliminate Sickle Cell Disease, marking a significant milestone in public health and genomic medicine.
TOP INSIGHT
Did You Know?
India's new #CRISPR breakthrough could turn Sickle Cell Disease into a treatable condition.
Gene Therapy, CRISPR Technology, and Sickle Cell Disease<
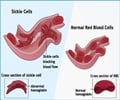
Gene therapy is a medical approach that uses functional genes to treat, prevent, or correct diseases by adding healthy copies or replacing faulty or missing ones in a patient's cells. CRISPR, short for Clustered Regularly Interspaced Short Palindromic Repeats, is a precise genome-editing system that allows targeted modification of DNA through two components: a guide RNA that identifies the exact genetic location to be altered, and an enzyme that cuts the DNA like molecular scissors.Sickle Cell Disease is an inherited condition that damages hemoglobin, causing red blood cells to become stiff and sickle-shaped, restricting blood flow and leading to severe complications, with India's tribal communities disproportionately affected, including an estimated one in eighty-six births among Scheduled Tribe populations.
Vision for National Elimination and Scientific Self-Reliance
The Minister noted that with the development and transfer of India's first indigenous CRISPR-based gene therapy, the nation is moving closer to fulfilling Prime Minister Narendra Modi's goal of creating a Sickle Cell–Free India by 2047, while simultaneously advancing the broader aspiration of Atmanirbhar Bharat in advanced medical innovation.Dr. Jitendra Singh highlighted that the breakthrough created at the CSIR–Institute of Genomics and Integrative Biology demonstrates India's ability to produce pioneering therapies at a small fraction of global prices, potentially replacing international treatments valued at ₹20–25 crore. He stressed the deep national importance of this achievement, particularly for tribal communities in central and eastern regions where the disease has the highest prevalence.
National Scientific Leadership and Gene-Editing Innovation
The ceremony included participation from senior leaders in Indian science, such as Dr. N. Kalaiselvi, Director General of CSIR; Dr. Souvik Maiti, Director of CSIR-IGIB; Dr. Umesh Shaligram, Executive Director of Serum Institute of India; as well as scientists, researchers, faculty, guests, and media representatives.Addressing the audience, Dr. Jitendra Singh said the indigenous CRISPR platform, named BIRSA 101 in tribute to Bhagwan Birsa Munda, marks a major scientific advancement that places India among global pioneers in sophisticated therapeutics.
Describing the gene-editing system in accessible terms, he explained that the technology operates like a “precise genetic surgery,” capable of not only treating Sickle Cell Disease but also reshaping treatment methods for various hereditary conditions.
Industry Collaboration Driving Gene Therapy Innovation
Dr. Jitendra Singh commended the increased collaboration between public scientific institutions and Indian industry, especially the Serum Institute of India. He stated that such partnerships have already delivered internationally recognized results, including vaccines for COVID-19, HPV, and other key diseases and will now accelerate India's role in gene therapy innovation.He emphasized that government alone cannot manage the entire expansion of biotechnology and that industry involvement is necessary for scale, cost-effectiveness, and global competitiveness.
During the visit, Dr. Jitendra Singh opened a new advanced research and translational center at CSIR-IGIB. He met with scientists, assessed work across genomic medicine programs, and emphasized the value of integrated national frameworks such as One Week–One Theme, which promote unified research efforts across CSIR, DBT, and allied institutions.
Technology Transfer and Scalable Gene Therapy Development
A formal agreement for technology transfer and collaboration was completed between CSIR-IGIB and Serum Institute of India Pvt. Ltd., enabling the transformation of IGIB's engineered enFnCas9 CRISPR platform into scalable and affordable treatments for Sickle Cell Disease and other key genetic disorders.Dr. Singh remarked that this collaboration mirrors India's effective public-private model that contributed to the creation of numerous vaccines and therapeutics over the past decade. He pointed out that such cooperation guarantees that scientific advances achieved in laboratories progress toward real clinical use at scale.
The Minister said that transferring BIRSA 101 and the CRISPR platform to a global leader like the Serum Institute ensures affordability, large-scale manufacturing, and internationally aligned production processes, ultimately making advanced gene-editing therapies accessible to Indian patients, especially those in underserved tribal regions.
India's Scientific Momentum and Global Positioning
Dr. Jitendra Singh concluded by stating that these breakthroughs reflect India's expanding scientific strength, evident in the swift development of vaccines, antibiotics, and now gene therapies. He called on scientific institutions, private sector partners, and policy organizations to adopt a unified One Week–One Theme approach to accelerate progress and ensure ongoing visibility for India's scientific achievements.Commitment to Accessible Gene Therapy
Speaking during the event, Dr. Umesh Shaligram, Executive Director of the Serum Institute of India, expressed deep appreciation and reaffirmed the organization's dedication to translating IGIB's innovation into meaningful real-world impact. He remarked:“Globally, gene therapies cost over three million dollars and remain inaccessible even for wealthy individuals. Our mission is to take Indian innovation and make it reachable for the poorest of the poor. Serum has saved more than 30 million lives with affordable vaccines, and we are fully committed to supporting the Prime Minister's vision of a Sickle Cell–Free India by 2047. With the drive and guidance from the Minister, we will work relentlessly to turn this technology into life-saving treatment.”
He further noted that the Serum Institute will continue to collaborate closely with IGIB and CSIR to ensure that advanced therapies reach the communities that need them the most.
In conclusion, the introduction of BIRSA 101 marks a powerful step toward accessible and transformative treatment for Sickle Cell Disease in India. By combining scientific innovation, public-private collaboration, and a commitment to affordability, the initiative sets the foundation for a future where advanced gene therapies reach even the most underserved communities.
Reference:
- Union Minister Dr. Jitendra Singh launches India's first indigenous "CRISPR" based gene therapy for Sickle Cell Disease, which particularly affects India's tribal population - (https://www.pib.gov.in/PressReleasePage.aspx?PRID=2191740)
Source-Medindia
 MEDINDIA
MEDINDIA



 Email
Email