The FDA has advised healthcare professionals to avoid using prefilled needleless glass syringes of amiodarone and adenosine due to frequent malfunction.
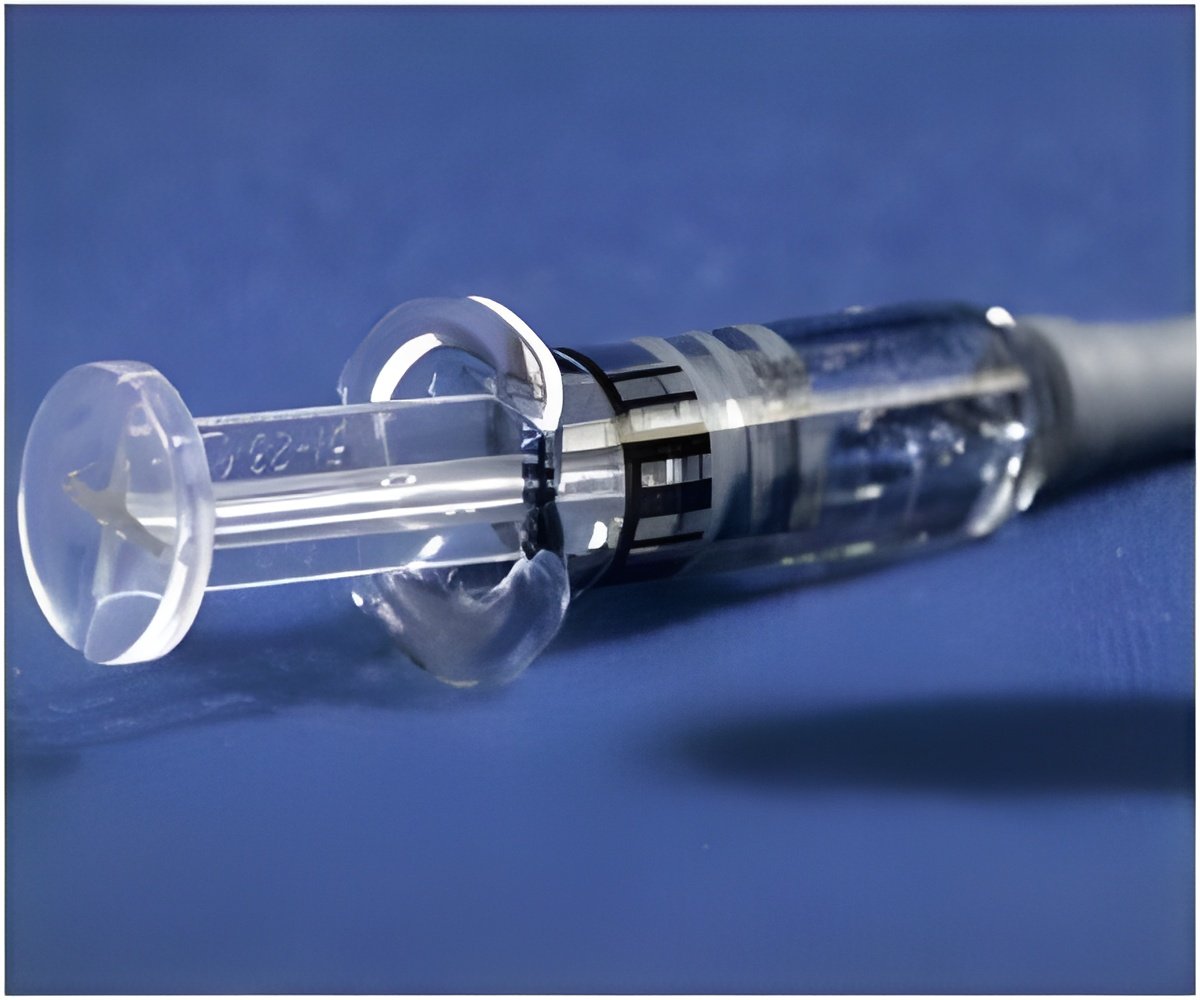
It has been observed that if the syringe and the access system are incompatible, the pin in the pin-activated IV access system can break off and block the syringe. This prevents the drug from being released into the IV line and results in a delay in administration of the drug. At times, the syringe damages the IV access system or the IV tube, necessitating a change in the access line. This could also further increase the time taken to deliver the medication. This delay is unacceptable for drugs like adenosine and amiodarone, which are used in life-threatening conditions like cardiac arrhythmias and have to be administered immediately during an emergency.
The FDA advises healthcare professionals to read the labels of the prefilled syringes carefully to make sure that they are compatible with the access system being used in the patient. It advises them to avoid using the prefilled glass syringes in emergencies.
The FDA also advises healthcare professionals to stock up enough vials or prefilled plastic syringes of these two drugs so that they can be used in lieu of the prefilled glass syringes in case of a malfunction.
Source: http://www.fda.gov/Drugs/DrugSafety/ucm254215.htm
Source-Medindia
 MEDINDIA
MEDINDIA

 Email
Email




