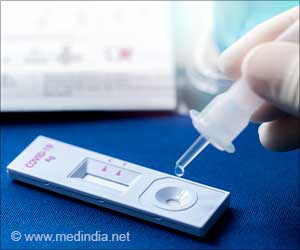
- FDA authorizes the emergency use of home testing kit that can differentiate between COVID-19 and flu
- The kit can produce the results within 30 minutes or less
- The test was developed by Lucira Health, a California-based company
At-Home, Over-the-Counter can Detect Both COVID-19 and Flu in 30 Minutes
With a shallow nasal swab, the single-use kit can provide results within 30 minutes indicating whether a person is positive or negative for COVID, as well as influenza A and influenza B, which are two common strains of the flu (1✔ ✔Trusted SourceFDA Authorizes First Over-the-Counter At-Home Test to Detect Both Influenza and COVID-19 Viruses
Go to source).
Who can Perform the Test?
People 14 and older can generally perform the test on themselves, the FDA says. Those between the ages of 2 and 13 can get results with the help of an adult. Dr. Jeff Shuren, the director of the FDA's Center for Devices and Radiological Health, said, "We are eager to continue advancing greater access to at-home infectious disease testing to best support public health needs". He also added that this is a major milestone in home testing.The test was developed by Lucira Health, a California-based company that was also the first to receive FDA approval for at-home rapid COVID tests back in 2020.
Accuracy of Test
According to the FDA, in people showing symptoms, the Lucira home kit accurately detected 88.3% of COVID infections and 90.1% of influenza A infections. The test can identify influenza B in lab studies, the FDA said. But because there are not enough cases of the virus circulating in real-world settings, further testing will be required, according to officials.Risk of False Positives or False Negatives
The FDA also warned that, similar to all rapid diagnostic tests, there is a risk of false positive and false negative results. The agency says individuals who test positive for COVID or the flu should take appropriate precautions and follow-up with a health care provider, while people who receive a negative result of either COVID or influenza B should confirm it with a molecular test preformed in a lab.Individuals who test negative but continue to experience symptoms of fever, cough or shortness of breath should also follow up with their health care provider in case of other respiratory viruses, the FDA said.
The dual-purposed test comes after a surge of COVID, the flu, and respiratory syncytial virus (RSV) that strained hospitals across the country last fall.
"The collective impact of COVID-19, flu and RSV underscore the importance of diagnostic tests for respiratory viruses," the FDA said in a statement.
Over the past few weeks, COVID-related deaths and hospitalizations have begun to fall, according to the latest data from the Centers for Disease Control and Prevention. Similarly, rates of flu and RSV-related hospitalizations have been going down, the CDC found.
Reference:
- FDA Authorizes First Over-the-Counter At-Home Test to Detect Both Influenza and COVID-19 Viruses - (https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-over-counter-home-test-detect-both-influenza-and-covid-19-viruses)
Source-Medindia