The German medical company revealed that series of laboratory tests had shown no evidence of contamination or other abnormality in their product used in Spain.
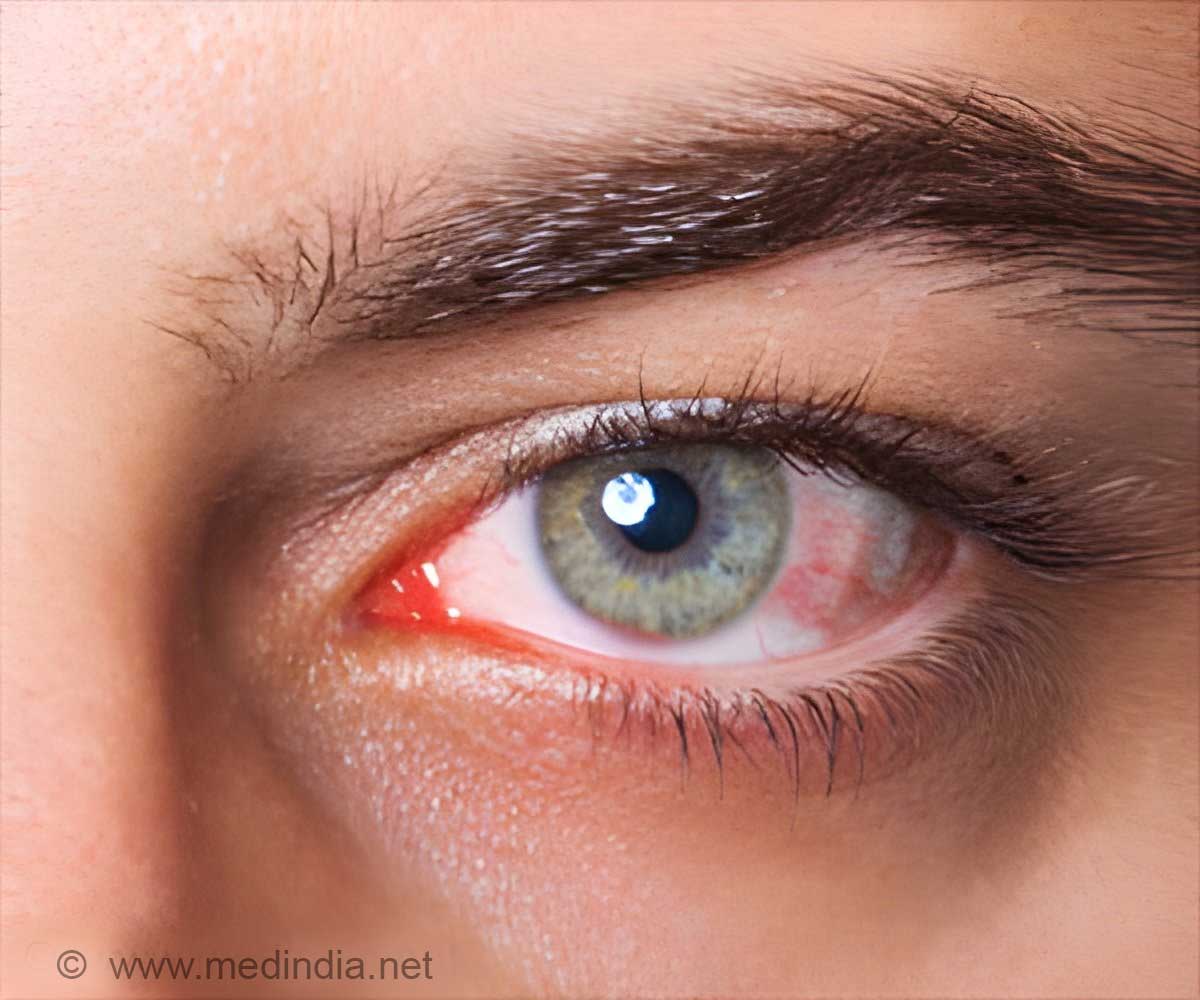
Spanish health authorities had recalled a compound Ala Octa manufactured by German company Alamedics, which is used in eye surgery. They alleged that the faulty substance has cost 13 Spaniards their sight in one eye and left 28 other patients also suffering injuries. However, the German medical company has rejected these claims.
Alamedics' general manager Christian Lingenfelder said, "A series of laboratory tests had shown no evidence of contamination or other abnormality in the product batch used in Spain. While there was an initial suspicion that such a medical product could have been the cause if contaminated, now the search (for the cause) must be conducted in the hospitals."
TOP INSIGHT
Laboratory tests have shown no evidence of contamination or other abnormality in Ala Octa. The company said that the product must be completely removed from the eye during surgery to avoid inflammation
Lingenfelder stressed the need for 'chemical purity' and 'correct application' of the product. He said, "The product must be completely removed from the eye during surgery to avoid inflammation, a fact that was known to specialist physicians and stated in the package leaflet."
The company said, "It was aware of at least one case in Spain where the product was left in the eye for one week instead of being immediately removed after surgery and urged further investigations."
Source-AFP
 MEDINDIA
MEDINDIA


 Email
Email




