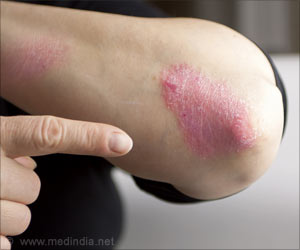
Numerous drug developments for atopic dermatitis (AD) that are under various clinical trials may completely transform the drug market of the disease over a decade.
TOP INSIGHT
Numerous drug developments for atopic dermatitis (AD) that are under various clinical trials may completely transform the drug market of the disease over a decade.
It is noted that almost 64% of the drugs in development for atopic dermatitis are small molecular agents and the rest 36% of the AD drug market under progress is contributed by biologics.
“The AD space has seen a dramatic rise in interest from the pharmaceutical industry, which is exemplified by the sheer size of its clinical development pipeline. An increasing number of companies are seeing the potential in this indication for its return on investment, and are entering the space singularly or through strategic partnerships. The introduction of unique drug classes to the AD space will provide patients with a plethora of options and will give doctors more choice although this may come with further scrutiny especially for the JAK class,” says Ramla Salad, a Pharma Analyst at GlobalData.
The Great Leap of Progress
The data notes that various products are under clinical development at different stages within the seven major markets (7MM*) for AD. This may mark huge progress in the treatment efficacy of the disease.
The small molecule agents include multiple late-stage JAK inhibitors (AbbVie’s Rinvoq – upadacitinib, Pfizer’s abrocitinib and Asana Bioscience’s gusacitinib) and PDE4 inhibitors (Otsuka, Medimetriks’ difamilast and Arcutis Biotherapeutics’ Pharmaceutical/ roflumilast) for topical use in AD.
All these biologics are administered subcutaneously. Among various drugs, Dupixent marks the highest uptake in AD with global reported sales of over $4bn in 2020.
Other Drugs in Pipeline
The other advancements in the drug advancement for AD include:
- The biologic OX40 inhibitors and small molecule sphingosine 1-phosphate receptor (S1PR) modulators – ground-breaking development.
- Kyowa Kirin/Amgen’s KHK-4083 for Phase III assessment, Kymab’s KY-1005 and Ichnos Sciences’ telazorlimab under Phase II trials.
- New oral therapy that includes Arena Pharmaceutical’s etrasimod for Phase the – first-in-class agent.
Source-Medindia
 MEDINDIA
MEDINDIA


 Email
Email