Researchers have developed a new 3D-printed medical device capable of accurately detecting specific bacteria responsible for Urinary Tract Infections (UTIs).

TOP INSIGHT
The newly developed 3D Medical Device enables early-stage detection of urinary tract infections (UTIs) caused by Klebsiella pneumoniae bacteria. #UrinaryTractInfections #Nanoparticle #KidneyDisease
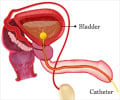
Urinary Tract Infection
Go to source). In many rural areas, UTI cases remain undetected due to a lack of adequate infrastructure, high cost of testing, and time. Hence, it is essential to identify the specific pathogen responsible for UTI for appropriate treatment.
Now, researchers described a generic approach to the fabrication of a prototype for the non-invasive detection of a specific pathogen using a tailor-made plasmonic aptamer-gold nanoparticle (AuNP) assay.
IIT Guwahati Makes Breakthrough in Urinary Tract Infections (UTI) Detection
The new Medical Device ensures rapid identification of the bacteria causing UTIs within just five minutes from a patient’s urine sample. This swift detection enables timely treatment, reducing the patient’s suffering and mitigating the risk of severe complications that might arise from untreated UTIs.The estimated manufacturing cost of the Medical Device is an affordable Rs 608, and testing a single sample costs only Rs 8. This cost-effectiveness makes the medical device accessible to a broader range of healthcare facilities, potentially benefiting a larger segment of the population.
The research findings explaining the Point-Of-Care testing prototype have been published in the journal, ACS Applied Bio Materials.
The conventional method of diagnosing UTIs through urine culture can take up to two days, causing significant delays in administering appropriate antibiotics. This delay poses a serious problem as untreated UTIs can lead to life-threatening situations.
Specific Instantaneous Detection of Klebsiella pneumoniae for UTI Diagnosis with a Plasmonic Gold Nanoparticle Conjugated Aptasensor
Go to source).
Therefore, the device’s ability to identify this bacterium quickly is of critical importance in providing timely and life-saving treatment. This device employs gold nanoparticles with engineered aptamers to detect the target bacteria accurately.
This unique mechanism facilitates fast and accurate detection, benefiting primary healthcare significantly. With successful validation against hospital results, the technology is now poised for optimization and commercialization, aiming to benefit society.
The development of the fast, accurate, and affordable Medical Device marks a major advancement in medical technology. By enabling early-stage detection of UTI-causing bacteria, the device promises to revolutionize UTI diagnosis and treatment, potentially saving lives and improving the overall healthcare landscape.
With strong collaboration and successful validation, the Medical Device is well-positioned for future commercialization and widespread use, serving the needs of countless patients in the coming years.
References:
- Urinary Tract Infection - (https://www.acpjournals.org/doi/10.7326/AITC201710030)
- Specific Instantaneous Detection of Klebsiella pneumoniae for UTI Diagnosis with a Plasmonic Gold Nanoparticle Conjugated Aptasensor - (https://pubs.acs.org/doi/10.1021/acsabm.3c00369)
Source-Medindia
 MEDINDIA
MEDINDIA




 Email
Email