Ixekizumab Medication Information
Discover comprehensive details about Ixekizumab, including its pronunciation, uses, dosage instructions, indications, and guidelines on how and when to take it or avoid it.
The updated prescription information covers potential side effects, precautions, warnings, and storage recommendations.
Additionally, explore the Ixekizumab brands available in India and internationally, along with pricing information. For personalized advice, consult your healthcare provider.
Generic Name : Ixekizumab Pronunciation : IX-ee-KIZ-ue-mab.Brand Names or Trade Names of Ixekizumab
India :
Taltz
Overview of Ixekizumab
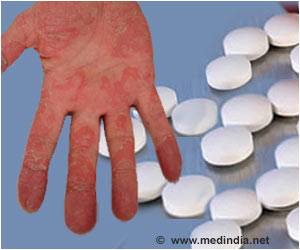
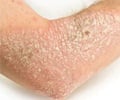
Ixekizumab is a medicine that affects the patient’s immune system. It is used to treat adults with moderate to severe plaque psoriasis who benefit from taking injections or tablets (systemic therapy) or phototherapy (treatment using ultraviolet or UV light).Why is Ixekizumab Prescribed? (Indications)
Ixekizumab injection, an interleukin antagonist is indicated for the treatment of adults with moderate-to-severe plaque psoriasis that have benefited from systemic therapy or phototherapy.When should Ixekizumab not be taken? (Contraindications)
Ixekizumab should not be used in patients:• If there is an allergy to ixekizumab
• Patients below 18 years
What is the dosage of Ixekizumab?
Ixekizumab injection is given subcutaneously by autoinjector and by prefilled syringe.Injection contains 80 mg/mL solution of ixekizumab.
The dose is 160 mg (two 80 mg injections) at Week 0, followed by 80 mg at Weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks for treating adult plaque psoriasis.
How should Ixekizumab be taken?
Ixekizumab is administered by subcutaneous injection. It should be given under the guidance and supervision of a healthcare professional.Patients may self-inject after taking proper training in subcutaneous injection technique using the autoinjector or prefilled syringe.
Injection should be taken at a different anatomic location (such as upper arms, thighs or any quadrant of abdomen) than the previous injection.
Ixekizumab should not be injected in the areas where the skin is tender, bruised affected by psoriasis. Injection in the upper, outer arm may be performed by a healthcare provider.
Before injection, remove Ixekizumab autoinjector or prefilled syringe from the refrigerator and allow it to reach the room temperature (30 minutes) without removing the needle cap.
Inspect ixekizumab solution visually for any particulate matter and discoloration prior to administration. It is clear and colorless to slightly yellow solution.
Do not use if the injection contains visible particles, is discolored or cloudy (other than clear and colorless to slightly yellow).
Any unused product left in the autoinjector or prefilled syringe should be discarded.
Patients are instructed to inject the full amount (1 mL), which provides 80 mg of ixekizumab
Missed a Dose?
If a dose of ixekizumab is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regularly scheduled time.What are the warnings and precautions for Ixekizumab?
•Ixekizumab may lower the capability of the immune system to fight infections and may increase the risk of infections, which can sometimes become serious.• Your doctor should check you for tuberculosis (TB) before starting the treatment with ixekizumab
• Inform your doctor if you have a past history of TB.
• Ixekizumab should not be used until any active infection subsides.
• Inform your doctor if you suffer from ulcerative colitis or crohn’s disease.
What are the side effects of Ixekizumab?
Gastrointestinal: NauseaRespiratory: Upper respiratory tract infections
Skin: Tinea infections
Others: Injection site reactions
What are the other precautions for Ixekizumab?
Caution is needed in case of usage of Ixekizumab in pregnant women.In case of overdosage, monitor the patient for any signs or symptoms of side effects and appropriate symptomatic treatment should be taken immediately
 MEDINDIA
MEDINDIA
 Email
Email