Fremanezumab-vfrm Medication Information
Discover comprehensive details about Fremanezumab-vfrm, including its pronunciation, uses, dosage instructions, indications, and guidelines on how and when to take it or avoid it.
The updated prescription information covers potential side effects, precautions, warnings, and storage recommendations.
Additionally, explore the Fremanezumab-vfrm brands available in India and internationally, along with pricing information. For personalized advice, consult your healthcare provider.
Generic Name : Fremanezumab-vfrm Pronunciation : free-ma-NEZ-ue-mab Therapeutic Classification : Monoclonal antibodiesBrand Names or Trade Names of Fremanezumab-vfrm
India :
Ajovy
Why is Fremanezumab-vfrm Prescribed? (Indications)
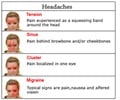
Fremanezumab-vfrm is prescribed for the preventive treatment of a migraine headache in adults.Fremanezumab-vfrm is a genetically engineered monoclonal antibody that works by blocking the actions of specific chemicals called calcitonin-related polypeptides, thereby reducing the frequency of migraines.
When should Fremanezumab-vfrm not be taken? (Contraindications)
Fremanezumab-vfrm should not be used in patients-• If there is an allergy to fremanezumab-vfrm
• Children
Caution is required when using fremanezumab-vfrm in pregnant and breastfeeding women to avoid the risk caused to the fetus or the baby.
What is the dosage of Fremanezumab-vfrm?
The recommended dose of fremanezumab-vfrm can be selected based on the following two dosing options-• 225 mg monthly or
• 675 mg quarterly (once every three months) given as three injections of 225 mg
When switching between the dosing options from once a month to once every three months or vice versa, it is always safe to give the first dose of the new regimen as per the old schedule and then follow the new schedule.
How should Fremanezumab-vfrm be taken?
• Fremanezumab-vfrm comes as a prefilled syringe which should be given as a subcutaneous injection (under the skin).• The injection sites include areas of the abdomen, upper arm or the thigh and the sites can be switched each time when an injection is given.
• Never inject into thickened, scaly, tender, damaged, red or bruised skin.
• Fremanezumab-vfrm injections should be given only by a healthcare professional who is properly trained in administrating the prefilled syringe.
• While giving 675 mg dose of fremanezumab-vfrm injection, use three separate injections of 225 mg that can be given in the same site but not in the same spot.
• Before injecting, it is necessary to keep the fremanezumab-vfrm vial outside the refrigerator and allow it to come to room temperature for at least 30 minutes.
• Placing the prefilled syringe in a bowl of hot water or in a microwave oven with the intention of warming the refrigerated prefilled syringe is not advised.
• It is generally advised to inspect the contents of the vial for the presence of any solid or visible particles and discoloration or any change in appearance. Discard the vial contents if such things are present.
Missed a Dose?
• If a dose of fremanezumab-vfrm is missed, take the missed dose as soon as possible.• If a missed dose is 225 mg, then take the next dose after one month from the last dose.
• If a missed dose is 675 mg, then take the next dose after three months from the date of the last dose.
• Never take a double dose of fremanezumab-vfrm to make up for the missed dose which can be harmful.
What are the warnings and precautions for Fremanezumab-vfrm?
• Patients can experience severe allergic reactions or hypersensitivity such as rashes, itching or urticaria during the treatment with fremanezumab-vfrm. If such allergies occur, patients are advised to consult the physician.• Steroid treatment can be advised for patients reported with allergies. The treatment with fremanezumab-vfrm can even be discontinued if the allergic reactions are severe or life-threatening.
• Caution is required if fremanezumab-vfrm is used in patients with a history of severe liver or kidney disease.
What are the side effects of Fremanezumab-vfrm?
The most common side effects of fremanezumab-vfrm include-• Injection site reactions (pain, swelling, and itching)
• Allergic reactions such as swelling of the face or the throat and breathing difficulty
What are the other precautions for Fremanezumab-vfrm?
• Use the vial within 24 hours if kept at room temperature and discard if it is kept for more than 24 hours.• Never take a fremanezumab-vfrm injection for a condition it is not prescribed.
• Do not recommend fremanezumab-vfrm to another person with similar symptoms of a migraine because it requires medical judgment.
What are the Drug Interactions of Fremanezumab-vfrm?
• Administering fremanezumab-vfrm along with other medications or giving other drugs in the same injection site of fremanezumab-vfrm should be avoided.• The doctor should be informed if the patient is currently taking or is planning to take any medications or herbal supplements before prescribing fremanezumab-vfrm to avoid dangerous side effects caused by the drugs.
What are the storage conditions for Fremanezumab-vfrm?
• Store the fremanezumab-vfrm vials in a refrigerator between 2°C and 8°C.• Keep the vials their original carton and protect from extreme heat or direct sunlight.
• Do not shake or freeze the vial contents.
• Keep the fremanezumab-vfrm prefilled syringe away from the reach of children.
 MEDINDIA
MEDINDIA
 Email
Email