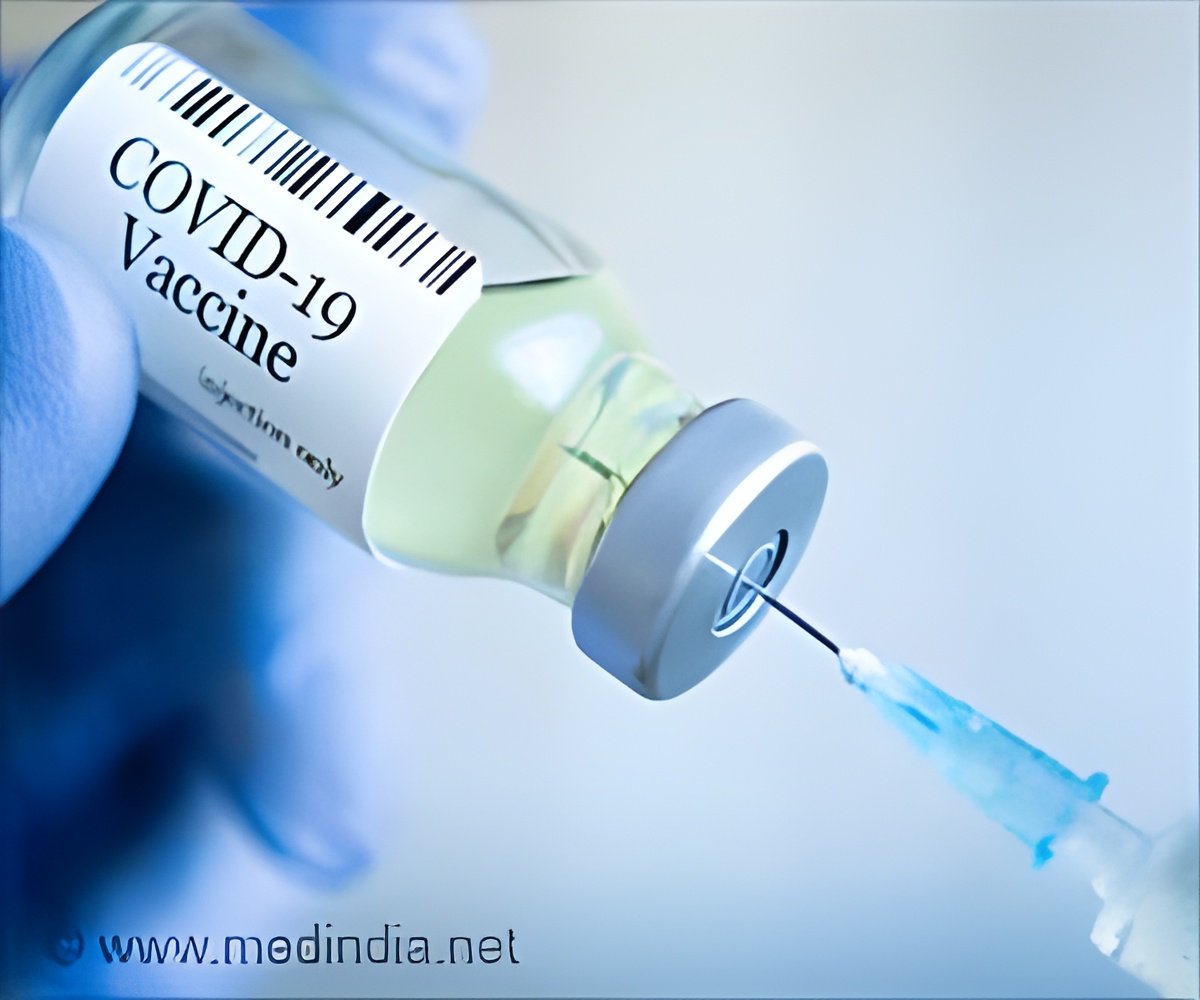
Merck & Co, the US pharmaceutical giant has announced that it is discontinuing development of its Covid-19 vaccine candidates -- V590 and V591 -- after they failed to generate immune responses.
TOP INSIGHT
Merck now plans to focus its Covid-19 research strategy and production capabilities on advancing two therapeutic candidates -- MK-4482 and MK-7110.
MK-7110 (formerly CD24Fc) is a potentially first-in-class investigational recombinant fusion protein that modulates the inflammatory response to SARS-CoV-2, principally by targeting a novel immune pathway checkpoint, the company said.
Interim results from a Phase 3 study showed a greater than 50 per cent reduction in the risk of death or respiratory failure in patients hospitalized with moderate to severe Covid-19.
Full results from this study are expected in the first quarter of 2021.
In December, Merck announced a supply agreement with the US government to advance the manufacturing and initial distribution of MK-7110.
Due to the discontinuation of two potential Covid-19 vaccines, the company will record a charge in the fourth quarter of 2020. The charge will be included in Merck's generally accepted accounting principles (GAAP) results, but will not impact non-GAAP results.
In addition to advancing the development and production of MK-7110 and MK-4482, Merck will continue to conduct Covid-19 research.
Source-IANS
 MEDINDIA
MEDINDIA


 Email
Email