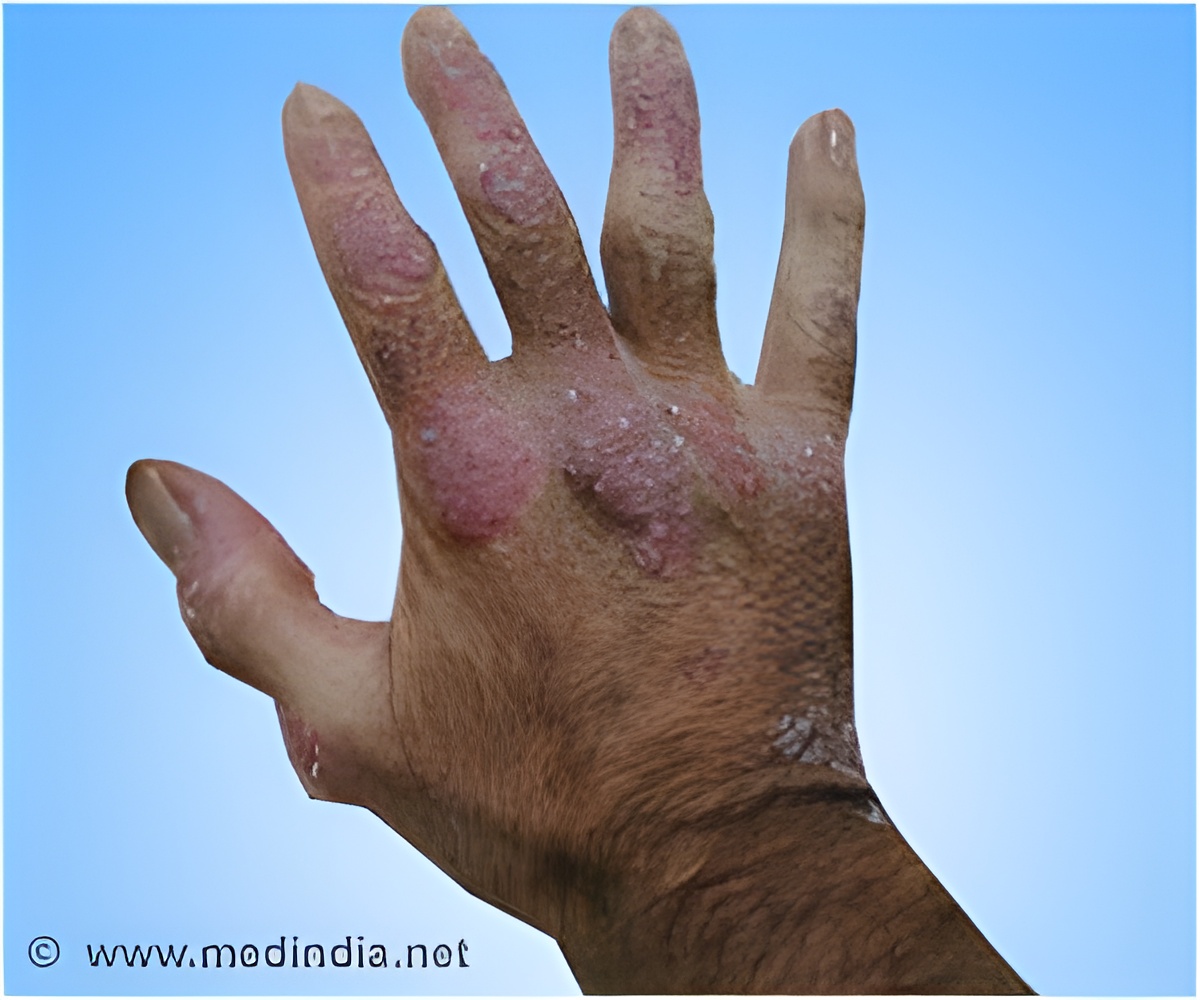
Lipidor AB gave an update on the Phase III study of the drug candidate AKP02 for the treatment of psoriasis.
Lipidor applied to the Indian Medical Products Agency for initiation of the Phase III study for AKP02 during early 2021. The aim of the study is to compare the effectiveness of AKP02 with existing drugs for psoriasis. Results are expected in the first half of 2022.
AKP02 is a drug candidate for psoriasis that contains calcipotriol and betamethasone. The drug is patient-friendly, spray-based treatment for mild to moderate plaque psoriasis.
TOP INSIGHT
New Phase III trial compares AKP02 with Enstilar. The trial will be done on 294 patients at circa ten clinics in India.
Read More..
Lipidor's AKVANO® technology works well for the treatment of psoriasis, as per the positive results obtained till now. Betamethasone also found to work well in AKVANO®, which is a good prerequisite for the combination preparation AKP02.
This Phase III study will involve 294 patients and is being conducted at circa ten clinics in India by Cadila Pharmaceuticals, a leading Indian pharmaceutical company with extensive CRO capacity.
Cadila said that they can effectively recruit patients, administer dosage and report clinical study results.
AKP02 will be compared with Enstilar. To recruit more patients, the scalp has also been included in the study. Psoriasis on the scalp can consist of anything from weak to severe scale formation and cause varying degrees of itching.
According to the Swedish Psoriasis Association, it is estimated that circa 60-70 percent of people who have psoriasis also get it on their scalp. In some cases, psoriasis is found only on the scalp.
"The experience from AKP01 means that we also have high hopes for a successful study with our other psoriasis candidate, AKP02. It is gratifying that the market has shown a great interest in AKP02, and by financing the implementation of the Phase III study, Lipidor retains a larger upside of any outlicensing," says Ola Holmlund, CEO of Lipidor.
"We continue to see great potential in our first drug candidate AKP01 and we consider the opportunity to outlicense AKP01 and AKP02 in a single package to be an advantage for the licensee in registration, manufacture and commercialization."
Lipidor is closely monitoring developments and taking measures to minimize or eliminate the impact on the company's operations. To date, the COVID-19 pandemic has had a limited effect on Lipidor's operations, but the company may need to revise its schedules if the pandemic is prolonged.
Source-Medindia
 MEDINDIA
MEDINDIA

 Email
Email