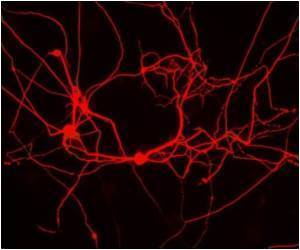
Evolution driven design Christof Fellmann, Johannes Zuber and coworkers at Cold Spring Harbor Laboratory (CSHL), USA, came up with strategies to improve RNAi technology when both were still working there. "The molecular underpinnings of efficient gene silencing are yet to be fully understood. Potent RNAi triggers are rare and have to be identified among hundreds to thousands of possibilities for each gene.
To advance current techniques, we looked at the evolutionary conservation of natural RNAi triggers to build enhanced synthetic analogues", Fellmann describes their approach. He continued to evolve this concept at Mirimus, a spin-off company from CSHL, while Zuber went on to found his own lab at the IMP, Austria. One particularly powerful RNAi method pioneered among others by Gregory Hannon and Scott Lowe at CSHL relies on embedding synthetic short hairpin RNA (shRNA) sequences into naturally occurring microRNA backbones. The resulting RNA molecules mimic natural triggers and are processed by cell intrinsic pathways. Yet, the efficiency of current reagents designed in this manner remains limited.
Fellmann and his team analyzed a specific microRNA backbone across various species, including opossum, chicken, elephant, rat and human, to identify sequence motifs that remain unchanged, indicating possible functional importance. The researchers then found that some of these sequences had been modified in one of the most commonly used synthetic RNAi backbones. By inverting these sequence regions back to their natural form and establishing a new shRNA backbone termed "miR-E", Fellmann and his team succeeded in greatly enhancing the efficiency of synthetic RNAi tools.
Realizing the full potential of RNAi "This advancement is highly relevant to reduce to practice the great promise of RNAi for drug discovery and biomedical research", Fellmann summarizes. While current methods require laborious and lengthy testing of many predictions to find an RNAi trigger that is sufficiently potent, the optimized "miR-E" backbone drastically increases the success rate through better processing of the precursor molecules.
Importantly, the new miR-E backbone can easily be integrated into current technologies to improve high-throughput RNAi screens and RNAi-based mouse models of human disease. Looking forward, Fellmann's study will open a promising avenue for generating focused and genome-wide shRNA libraries that will truly cover each gene with multiple effective shRNAs and constitute a validated and versatile tool for high-throughput functional genetics in the post-genomic era.
Advertisement