By studying frogs and fish, whose eggs are fertilized and develop outside the mother, the scientists can now address their hypotheses in living intact embryos.
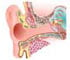
Our inner ear develops in the embryo from a simple flap of skin called the otic placode into a complex, three dimensional structure that enables balance and hearing.
The goal of the zebrafish research is to understand at the molecular level, how and why otic placode cells decide to become neuronal, non-sensory or sensory cells.
"Zebrafish provide a powerful, easily manipulated genetic system for understanding the role of specific molecules during development," said Collazo.
The main goal of the frog research is to determine which molecules and regions of the otic placode are required for normal patterning in the developing inner ear.
Advertisement
Collazo and his team discovered that physically removing either the front or back half of the otic placode in the Xenopus frog results in a high percentage of mirror image duplicated inner ears.
Advertisement
The studies also provide insights into some of the inner ear malformations seen in clinical patients.
Proper patterning, positioning and differentiation of the sensory organs within the inner ear are crucial for normal function in balance and hearing.
Studies have found that the gene mutations in zebrafish, which can result in mirror duplicated inner ears, are found in molecules belonging to the cell signaling pathway designated Shh.
Similarly, blocking the cell signaling pathway designated as Hh in the Xenopus frog or in zebrafish, results in two mirror image front halves and suggests that Shh signaling is necessary for patterning the back half.
This is important because any future therapies developed for replacing lost sensory cells (hair cells) that detect motion in the inner ear, will require that the regenerated hair cells be accurately placed and positioned.
Source-ANI