Previous research has shown that tumor cells fail to reach their potential in the wrong environment.
The enzyme, a specific isoform of a rather common kinase, may eventually become a target for cancer therapy as kinases constitute reasonably targetable enzymes, said Edna Cukierman, PhD, Associate Professor at Fox Chase, whose research focuses on the interactions between tumors and the microenvironments where they live. The research will be presented on April 6 at the AACR Annual Meeting 2014.
The microenvironment that Cukierman uses to help determine a cancer's fate is called the extracellular matrix (ECM), a network of structural molecules localized outside the cell that play a role in regulating numerous cell behaviors, for instance known by sending signals to promote wound healing or embryonic growth and development. Cukierman employs a three-dimensional model she developed to watch what happens when a tumor-altered matrix encourages cancer growth.
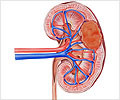
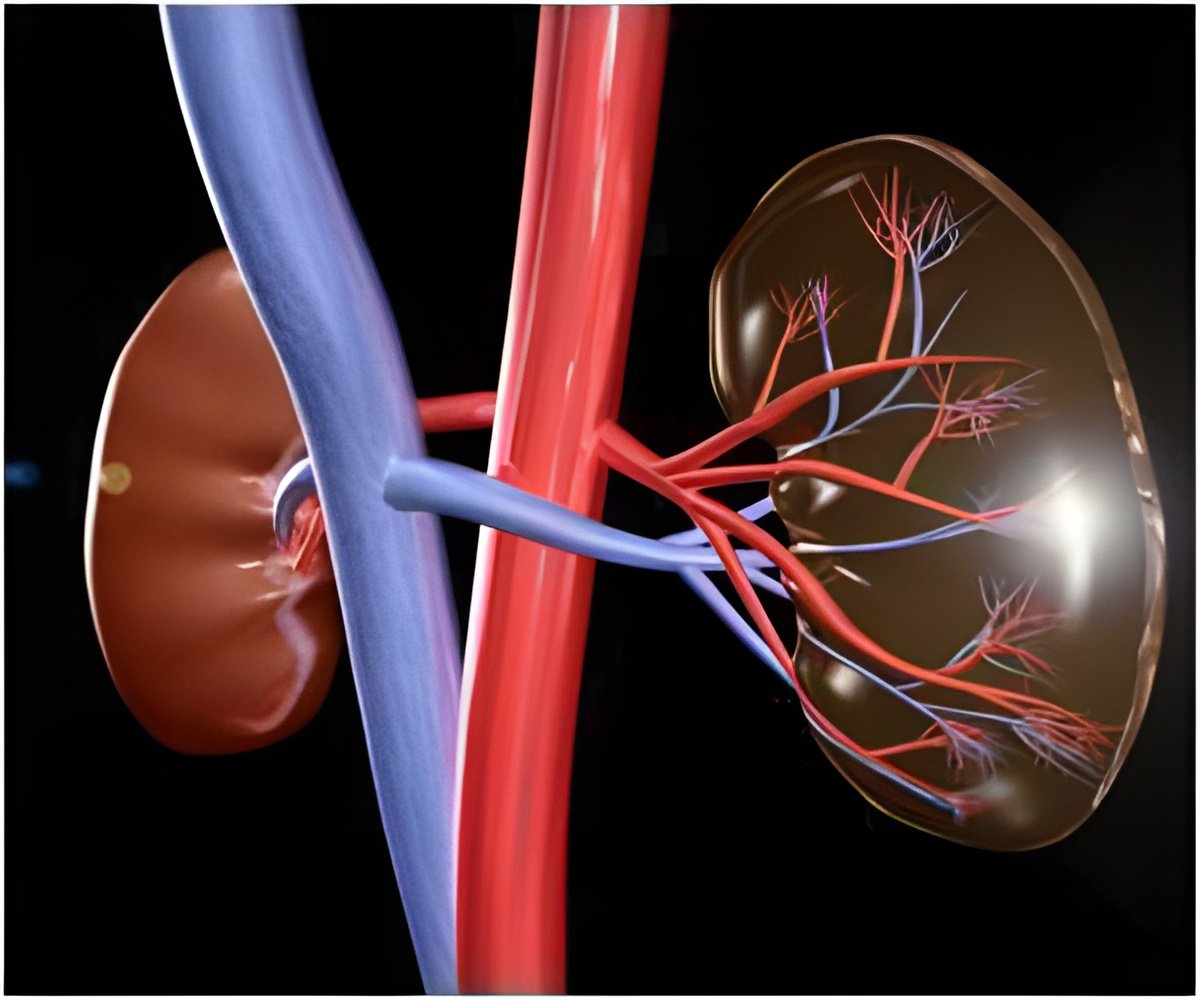
In cooperation with surgeons at Fox Chase, Cukierman uses freshly donated renal cell carcinoma tissue and extracts cells called fibroblasts from kidney tissue well outside of the tumor growth margins. She collects other fibroblasts from tissue within the cancer growth area of the same patient. The fibroblasts, which naturally secrete the mesenchymal extra cellular matrix, are used to create two sets of three-dimensional scaffolding per patient, one matrix from pro-growth fibroblast and a second from fibroblasts that keep tumor growth under control.
The researchers put renal cancer cells from established cell lines into both matrices. Those put in the tumor-promoting matrix proliferated quickly, with rapid metabolism and invasive capabilities. Identical cancer cells put in the normal matrix from the same patient grew slowly, failed to invade and when researchers withdrew external nutrients, the cells died. After the cancer cells were allowed to grow, the researchers looked at how the protein composition of the proliferating cancer cells differed from the lazy cancer cells by performing an RNA expression analysis. The analysis revealed that cancer cells in the pro-growth matrix had higher levels of RNA for a kinase normally found in neurons in the brain. A kinase is an enzyme capable of attaching phosphate groups to other molecules and many kinases constitute excellent targets used in cancer treatments.
When researchers inhibited the kinase, cancer cell division and spread halted, suggesting that the kinase was not just a cancer marker, but played an important role in cancer growth.
Advertisement
"This is really exciting because we know that we may be able to try, at some distant point in the future, ways to inhibit this enzyme. There's a good chance it will be rather specific to cancer. And since this kinase is otherwise found merely in the brain, we would try to make a drug that didn't cross the blood-brain barrier and create too many side effects," she said.
Advertisement
Source-Eurekalert