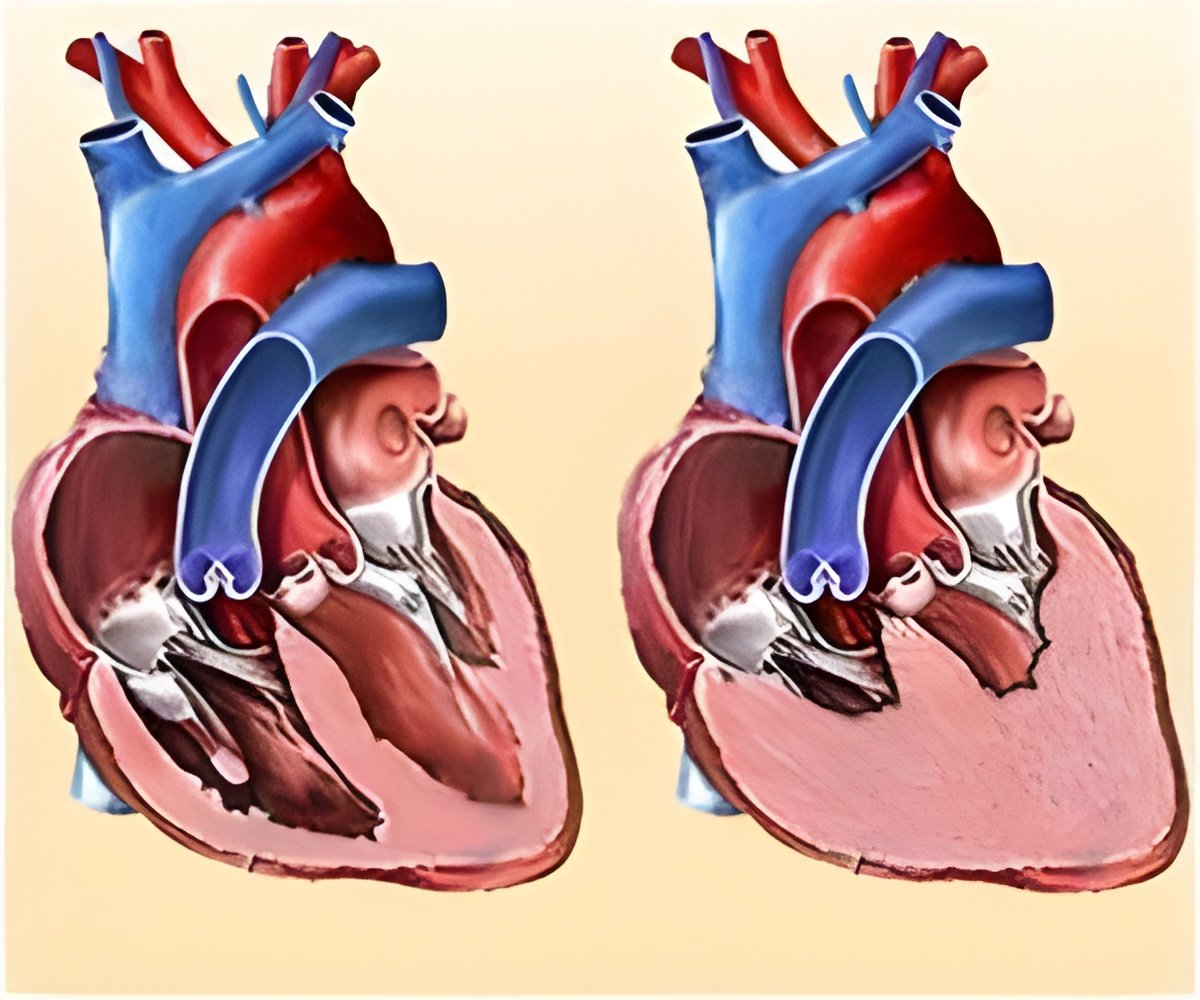
The FDA has issued a recall for Abbott's HeartMate 3 artificial heart devices, critical for heart transplant patients, due to safety concerns.
HeartMate 3 recall and inadequate hearts for transplantation make HF therapy landscape much more dire, says GlobalData
Go to source). Recently, the FDA announced a Class I recall for Abbott’s HeartMate 3 left ventricular assist device (LVAD), an MCSD that helps a failing heart pump oxygenated blood throughout the body. The shortage of hearts for transplantation and the HeartMate 3’s recall highlight the increasingly dire HF therapy landscape, according to GlobalData, a leading data and analytics company.
TOP INSIGHT
GlobalData reports over 7.8 million prevalent cases of #heartfailure in the US in 2023, with a compound annual growth rate (CAGR) of 2% from 2015 to 2023, driven by the aging population. #hearttransplant
Abbott’s Market Dominance in LVADs Post-2021
Before Medtronic discontinued its HeartWare LVAD System in 2021 due to high neurological adverse event rates, Abbott held about 58% of the LVAD market, with Medtronic at 42%. Since then, Abbott has maintained over 90% of the global LVAD market share.Carlos adds: "As the primary LVAD marketed in the US, the HeartMate 3 recall could be devastating for patients awaiting or ineligible for heart transplants."
Notably, this is not the first FDA recall for Abbott’s HeartMate 3. In 2018, a Class I recall was issued due to outflow graft testing occlusions post-implantation, leading to adverse effects like low blood flow or clotting. While LVAD alternatives are few, one promising substitute is Jarvik Heart’s Jarvik 2000.
Carlos concludes: "While market dominance benefits a company, the rising prevalence of HF underscores the need for more LVAD options. The Jarvik 2000 is currently being used in US hospitals for Bridge to Transplant Therapy in clinical trials."
- HeartMate 3 recall and inadequate hearts for transplantation make HF therapy landscape much more dire, says GlobalData - (https://www.globaldata.com/media/medical-devices/heartmate-3-recall-inadequate-hearts-transplantation-make-hf-therapy-landscape-much-dire-says-globaldata/)
 MEDINDIA
MEDINDIA
 Email
Email