Compared with adult cancer patients, parents of children with cancer were more likely to be dissatisfied with the informed consent process for participating in clinical trials,
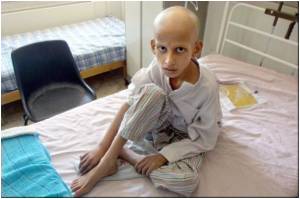
"These findings were consistent with what we suspected, given the different contexts in which adult and pediatric clinical trials are offered," said Steven Joffe, MD, MPH, senior author of the study. "But I didn't expect such large differences."
The researchers administered questionnaires to 47 parents and 204 trial participants within two weeks after the clinical study began.
Results showed that only 64 percent of parents felt they had enough time to learn about the trial, compared to 87 percent of adults. The parents were also less likely (79 percent to 93 percent) to report that they had sufficient opportunity to ask questions.
Even though they scored about the same on objective tests about the trials' particulars, parents of children with cancer rated themselves as less knowledgeable (79.5 percent) than did adult patients (87.8 percent).
Joffe, an ethicist and pediatric hematologist/oncologist at Dana-Farber/Children's Hospital Cancer Center, attributed much of the disparity to the differing contexts in which adults and pediatric patients are offered clinical trial participation.
Conversely, about three-quarters of the pediatric patients were facing a new diagnosis of cancer – usually a blood malignancy like leukemia.
Joffe said the study confirms concerns that have already prompted efforts to alleviate the confusion surrounding decisions made in the middle of an emotional crisis. For example, some medical personnel now make a return visit to patients or parents "after the initial storm has passed" to make sure they have understood the protocol correctly.
Also, clinical researchers, in some cases, are relieving clinical study recruits of the necessity to make decisions on all aspects of a trial right away, he said. "Often there are randomizations to one treatment or another that occur later in the overall trial, and we are delaying the consent process for those aspects rather than asking people to make all those decisions on Day One."
Source-Eurekalert
 MEDINDIA
MEDINDIA



 Email
Email










