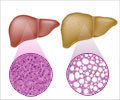
A human liver-immune organoid microarray reproduces patient-specific drug-induced liver injury and offers a scalable platform for predicting harmful immune responses to medications.
Autologous Organoid-T Cell Co-Culture Platform for Modeling of Immune-Mediated Drug-Induced Liver Injury
Go to source). This innovation, detailed in a study published online on September 26, 2025, in Advanced Science, involves a miniature liver system constructed from stem cells combined with a patient's immune cells.
It offers a powerful way to investigate why some individuals experience immune-related liver injuries from otherwise safe drugs. Fadoua El Abdellaoui Soussi, Ph.D., and Magdalena Kasendra, Ph.D., who are affiliated with the Center for Stem Cell and Organoid Medicine (CuSTOM) at Cincinnati Children’s, served as co-first author and corresponding author, respectively.
El Abdellaoui Soussi explained that the team aimed to develop a human model that mirrors how the liver and immune system function together in real patients. By incorporating patient-specific genetics and immune responses, the model offers insights into why certain medications only trigger liver injury in a small group of people.
TOP INSIGHT
Did You Know?
Only individuals carrying a specific gene may experience immune‑triggered liver damage from flucloxacillin, and this reaction was successfully reproduced using a personalized human liver‑immune organoid system. #medindia #liverorganoid #immuneinjury
Modeling Immune-Specific Liver Reactions
Some medications that initially pass safety evaluations can later cause idiosyncratic drug-induced liver injury, or iDILI, a rare immune response that may result in serious health issues or even lead to drug withdrawal from the market. Traditional laboratory tests and animal models fall short of capturing these complex immune interactions unique to individual patients.To overcome this, the research team combined liver organoids derived from induced pluripotent stem cells with each donor’s own CD8⁺ T cells, the immune cells that target infected or damaged cells. The result is a fully human, immune-competent model that reflects the genetic and immune diversity found in real-world populations.
Replicating Flucloxacillin-Induced Liver Damage
For validation, the team used their platform to replicate liver injury caused by flucloxacillin, an antibiotic known to trigger immune-related liver toxicity only in people who carry the HLA-B*57:01 risk gene.The model successfully mimicked key features of this immune-mediated damage, including T cell activation, cytokine release, and harm to liver cells, closely aligning with clinical observations in sensitive patients.
Kasendra emphasized that their long-term objective is to introduce human biological systems into the lab that are scalable and patient-relevant. She noted that by merging stem cell science with practical toxicology, this model brings organoid technology one step closer to reshaping drug development and safety testing.
Advancing Organoid Technology Through Stem Cell Innovation
This platform is built upon earlier breakthroughs by Takanori Takebe, M.D., Ph.D., a study co-author whose lab pioneered the production of human liver organoids from stem cells. The CuSTOM Accelerator team at Cincinnati Children’s adapted this foundation into a microarray system that eliminates the need for a matrix and incorporates immune cells matched to each patient, thus turning a research milestone into a precision toxicology tool.The project also illustrates a fruitful collaboration with Roche, whose role in translational toxicology was essential to advancing the work.
Adrian Roth, Ph.D., principal scientific director of Personalized Healthcare Safety at Roche, highlighted how the partnership merges academic innovation with industrial application. He remarked that together, the teams are developing predictive human models to enhance drug safety and speed up the delivery of new treatments.
Expanding the Impact of Organoid Science
Since 2010, Cincinnati Children’s has been at the forefront of organoid medicine, beginning with the creation of the first functional human intestinal organoids.Now, under Kasendra’s guidance, the CuSTOM Accelerator collaborates with biopharmaceutical and tech companies to turn these scientific advances into real-world applications focused on drug safety, targeted medicine, and regenerative treatments.
Automating Organoid Testing for Diverse Populations
The CuSTOM Accelerator team is now working on automating organoid testing and scaling it for high-throughput screening across diverse donor groups. This next step is crucial for fully capturing the broad range of human genetic variability, which will enable the development of treatments that are more effective, inclusive, and individually tailored.Kasendra concluded that this initiative reflects CuSTOM’s mission to transform organoid research into tools that make a real difference in healthcare. She stressed that this is only the beginning, by combining biology, engineering, and clinical insights, the team is advancing toward a future where drug responses can be accurately predicted before a treatment reaches a patient.
Reference:
- Autologous Organoid-T Cell Co-Culture Platform for Modeling of Immune-Mediated Drug-Induced Liver Injury - (https://pubmed.ncbi.nlm.nih.gov/41001778/)
Source-Eurekalert
 MEDINDIA
MEDINDIA



 Email
Email