The first large-scale trials of two Ebola vaccines, GlaxoSmithKline's Chad3-EBO-Z and Merck and Newlink's rVSV-ZEBOV, has begun in Liberia at the Redemption Hospital in the capital Monrovia.
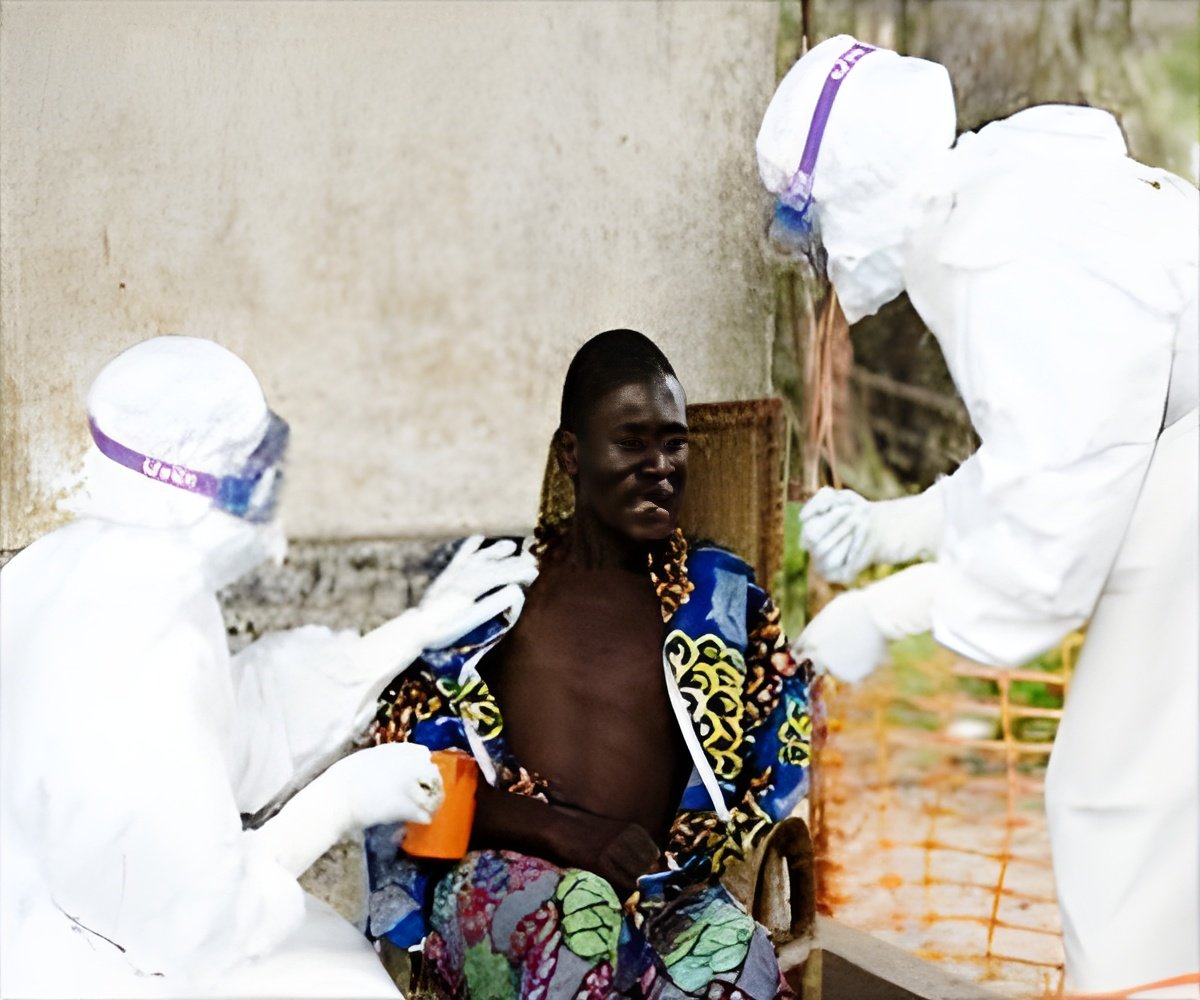
The Partnership for Research on Ebola Vaccines in Liberia (PREVAIL), which is a collaboration between the United States and Liberia, said that trials would begin at other hospitals around Monrovia after the first 600 participants join the study. PREVAIL said during the previous smaller trials in other countries it has been seen that the drugs could cause pain, redness or swelling in the injected arm, as well as fever, headaches and tiredness, but added that the side-effects typically have been mild to moderate and have gone away on their own.
President Boaikai said, "We hope that this scientific undertaking we launch here today will get answers for the mystery surrounding this disease."
Source-Medindia
 MEDINDIA
MEDINDIA

 Email
Email