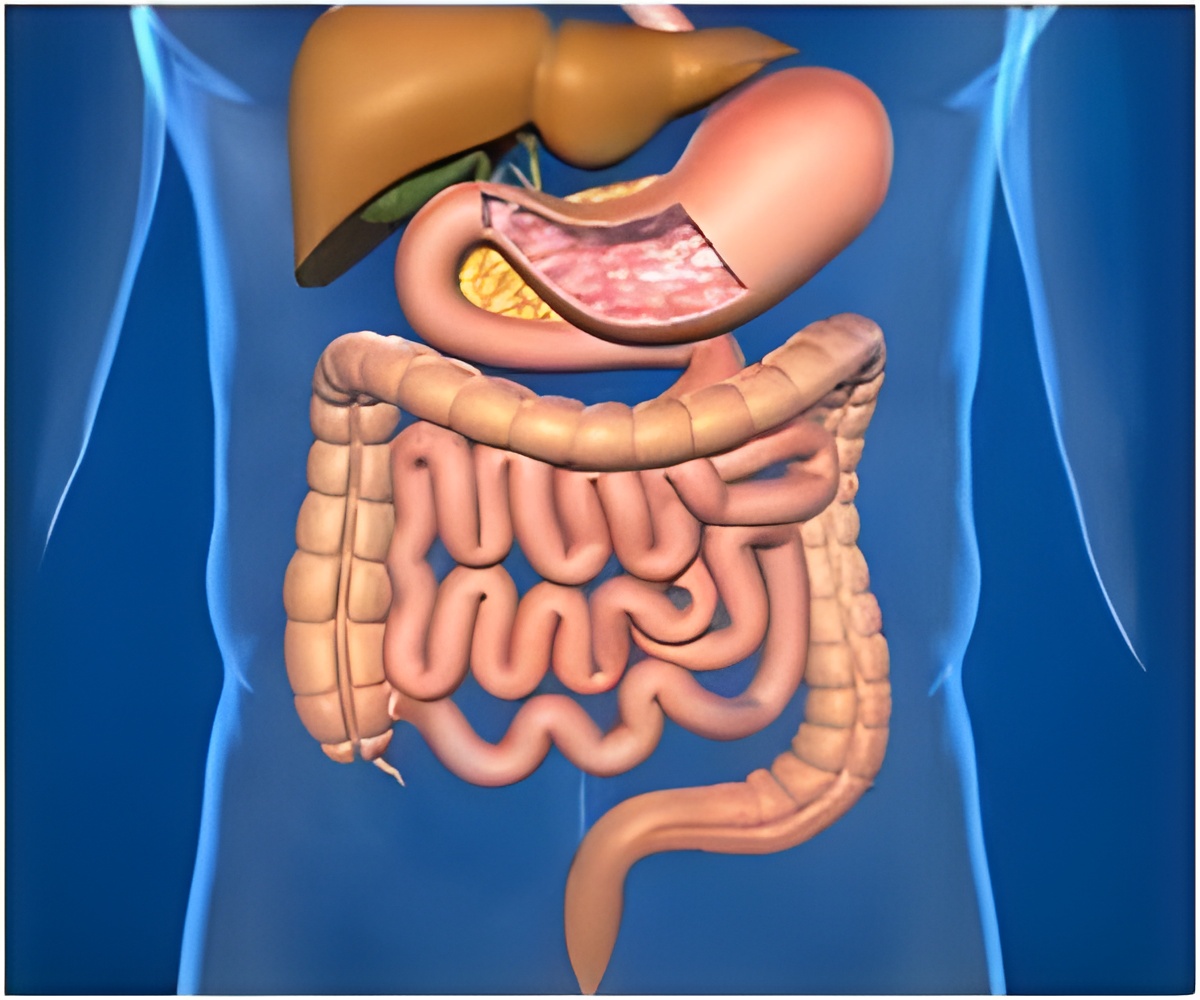
Bowel cancer is Australia's 2nd deadliest cancer, causing over 5,000 deaths annually.
'Game-changer' clinical trial launches for Australia's second-deadliest cancer
Go to source). The findings could help replace current trial-and-error treatment practices with a more tailored and personalized approach – improving the survival rates and quality of life for people living with bowel cancer.
TOP INSIGHT
Victoria leads the way in #bowelcancer research! A new #clinicaltrial launching across multiple hospitals has the potential to transform how bowel cancer is treated. #Australia
Organoid Breakthrough Underpins Revolutionary Bowel Cancer Clinical Trial
The trial is underpinned by a breakthrough study led by WEHI and partner hospitals in Victoria that found tumor organoids – 3D cancer models grown in the lab – can accurately predict what drugs will work for bowel cancer patients with advanced stages of the disease.The trial could transform current treatment selection practises for bowel cancer patients, improving their survival rates and quality of life.
While 99% of bowel cancer cases can be treated successfully if found early, less than half of all patients are diagnosed at the initial stages due to a lack of symptoms – making early intervention a challenge.
As there is currently no way to predict how a person with bowel cancer will respond to specific chemotherapy drugs, some patients may receive ineffective treatments.
Now a new clinical trial, FORECAST-2, is hoping to overcome this critical challenge by using tumour organoids – mini cancers grown in the lab from a patient’s own tissue samples.
Co-lead researcher, Professor Peter Gibbs, said the trial could revamp the trial-and-error processes that currently guide the treatment selection process for patients.
“Unfortunately, up to 40% of bowel cancer patients will develop advanced stages of the disease, requiring chemotherapy treatment.
“Given we now have many treatment options to select from, identifying which of these therapies to give a patient will ultimately have a big impact on their health outcomes.”
The size of a grain of sand, organoids can mimic the characteristics of the cancer from which they are created, including sensitivity to drug treatment.
Each patient tissue sample can be used to grow up to eight tumour organoids, which can then be tested with different drug combinations to determine a patient’s optimal treatment.
“Our research to date shows that if a drug has no effect on the organoid, then this treatment would also have no effect on the patient,” Prof Gibbs said.
“Knowing what is most likely to work before patients start treatment would make a significant difference to their survival outcome and quality of life.”
The trial is underpinned by a landmark WEHI-led study that was the first in the world to validate organoid drug testing as an accurate tool in the treatment selection process.
In the study, researchers pre-tested chemotherapy drugs on the organoids of 30 patients with advanced bowel cancer and found organoid drug testing could predict:
- the treatments that won’t work for the individual patient, with 90% accuracy.
- the treatments that will work for the individual patient, with 83% accuracy.
The new trial will look at whether those results can be replicated in people who have recently been diagnosed.
Associate Professor Oliver Sieber, a corresponding author on the original study and WEHI Laboratory Head, said FORECAST-2 could be a breakthrough in the future of personalised medicines.
“Every cancer is unique and requires a tailored treatment approach for the best outcome,” Assoc Prof Sieber said.
“Being able to predict the treatment outcomes for newly diagnosed patients will give us the best chance of identifying the most promising treatments early.
“It’s an incredibly exciting moment to see our results be translated into a clinical trial that we hope will become a game-changer for bowel cancer patients in Australia and around the world.”
Reference:
- ‘Game-changer’ clinical trial launches for Australia’s second-deadliest cancer - (https://www.wehi.edu.au/news/game-changer-clinical-trial-launches-for-australias-second-deadliest-cancer/)
Source-Eurekalert
 MEDINDIA
MEDINDIA


 Email
Email










