A breakthrough intranasal therapy for brain tumors enters expanded clinical trials in India.
NeOnc Technologies Holdings, Inc. Rings the Nasdaq Stock Market Opening Bell
Go to source).
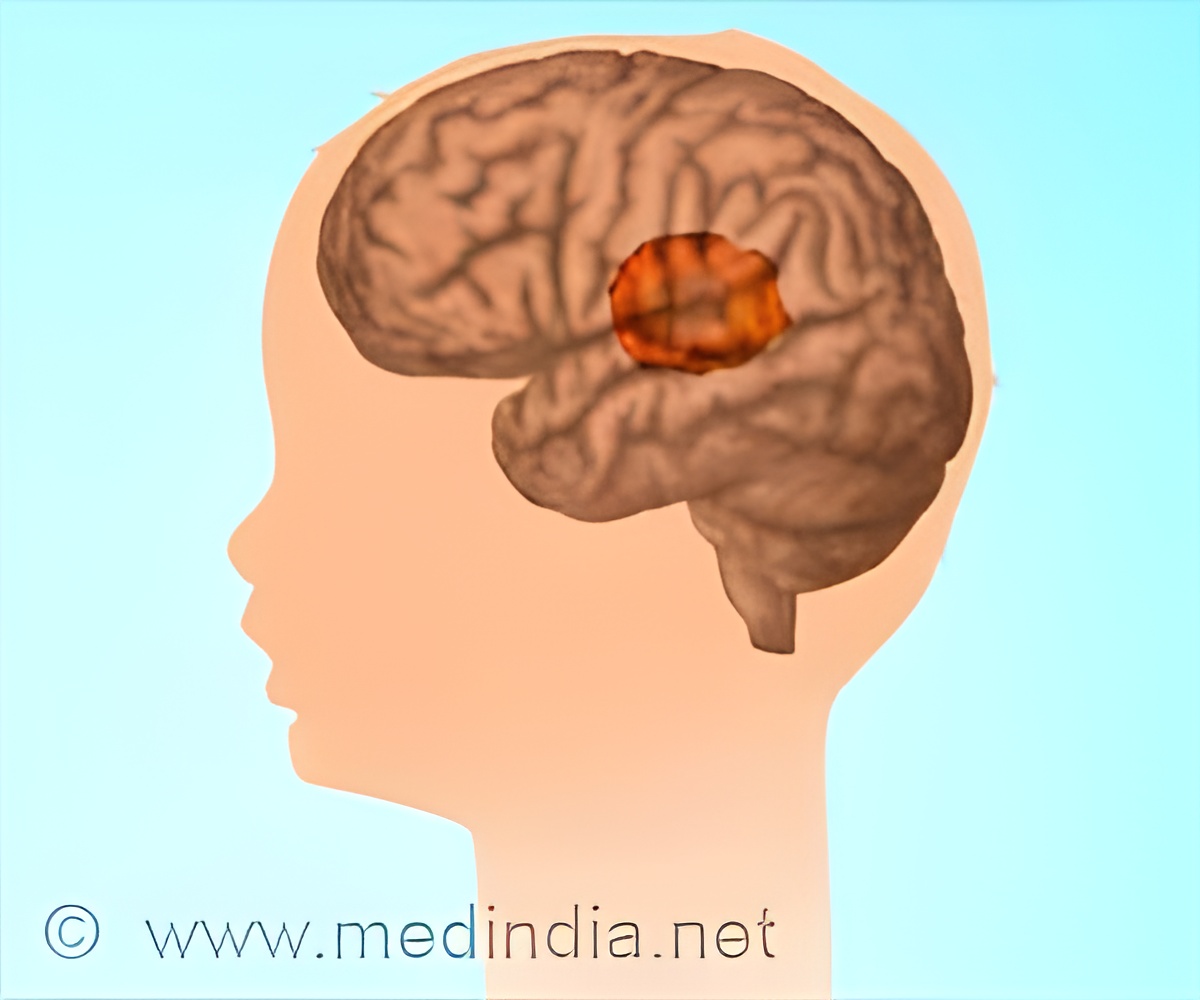
How the Intranasal Delivery System Works
The system delivers NEO100, an ultra-purified form of perillyl alcohol, through inhalation. The molecule enters the cerebrospinal fluid via the olfactory nerve, ensuring direct contact with tumor sites while minimizing systemic toxicity. Unlike traditional chemotherapy, which relies on oral or intravenous administration, this method allows for higher drug concentrations in the brain without significant side effects.TOP INSIGHT
Targeting brain tumors just got easier-bypass the blood-brain barrier with intranasal drug delivery. #braincancer #medindia
Phase 1 clinical trials have already demonstrated the safety and tolerance of this approach, and Phase 2 trials are now underway. To accelerate research, clinical trials are expanding across 30 FDA-compliant sites in India in collaboration with CBCC Global Research. This expansion will enhance patient recruitment, ensuring broader access to the treatment and expediting regulatory approvals.
Potential Applications Beyond Brain Tumors
Beyond brain tumors, this intranasal drug delivery system holds potential for treating other neurological conditions, such as pediatric brain tumors, metastatic brain cancer, and even neurodegenerative diseases like Alzheimer's. By proving that therapeutic agents can effectively reach the brain through intranasal administration, this method could revolutionize the way neurological diseases are treated.The success of this approach marks a critical advancement in neuro-oncology, offering new hope to patients facing limited treatment options. As clinical trials progress, this innovative system may pave the way for more effective and targeted therapies for a wide range of brain-related disorders.
Reference:
- NeOnc Technologies Holdings, Inc. Rings the Nasdaq Stock Market Opening Bell - (https://www.nasdaq.com/videos/neonc-technologies-holdings-inc-rings-nasdaq-stock-market-opening-bell)
Source-Medindia
 MEDINDIA
MEDINDIA



 Email
Email










