It is important to understand the mechanism of hemostasis and potential pro-inflammatory effects the nanoparticles might have on this process.
TOP INSIGHT
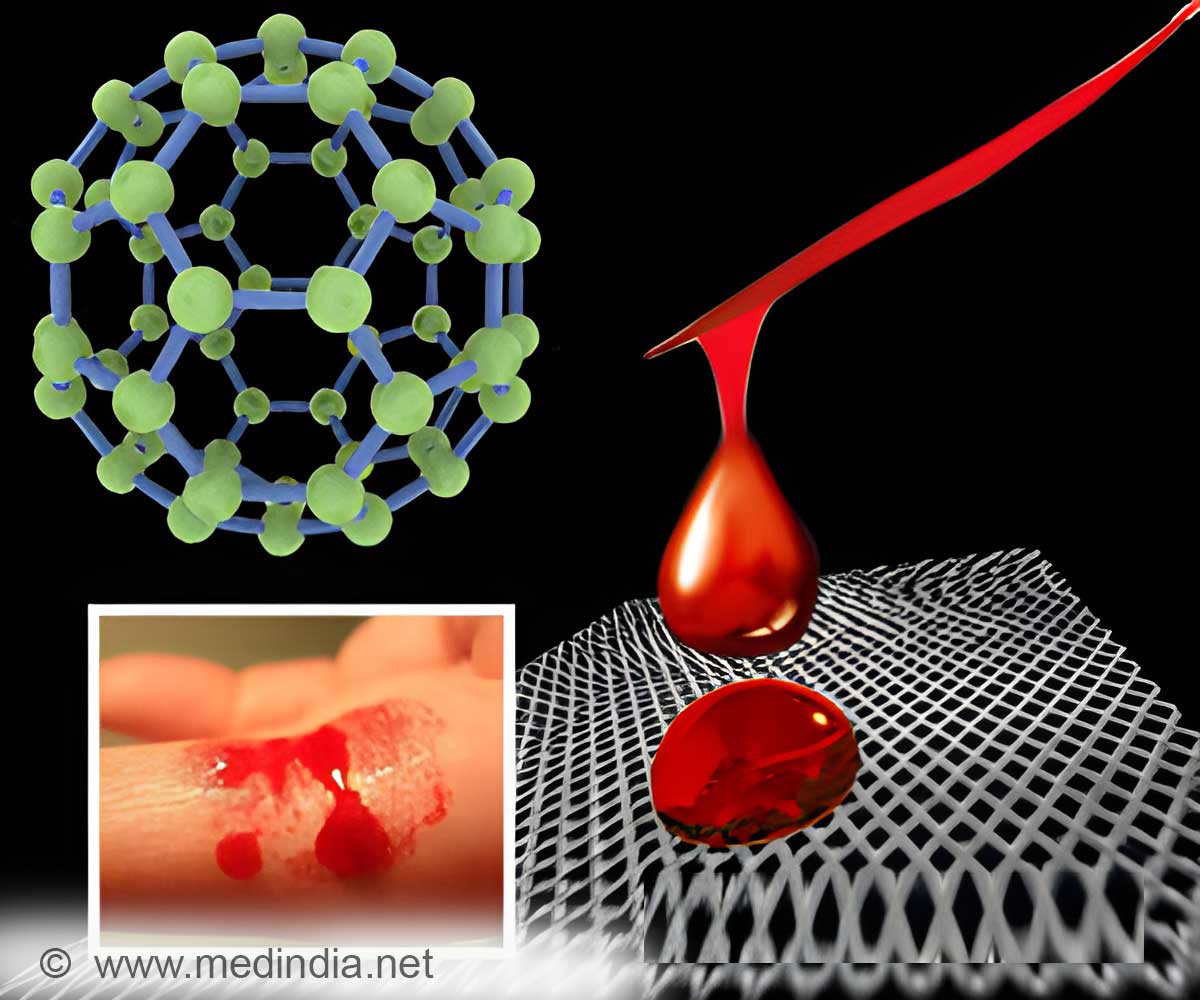
Exposure to nanoparticles deregulates the complex interplay between endothelial cells, plasmatic coagulation and platelets, and alters hemostasis.
It is important to understand the mechanism of hemostasis and potential pro-inflammatory effects the nanoparticles might have on this process. There are several in vitro assays available that, according to the presented in vitro-in vivo comparisons, can identify adverse actions of nanoparticles on hemostasis.
Particle producers should be aware of the advantages and limitations of specific in vitro assays for hemostasis and not restrict the testing of cytotoxicity. Use of in vitro assays allows the screening for new coating materials, which appear to be needed, because the commonly used polyethylene glycol was able to prevent interaction with clotting only by some particles.
This article illustrates the mechanism and regulation of hemostasis, provides information on nanoparticle action on hemostasis in vitro and in vivo and describes concept and limitations of in vitro assays in the assessment of nanoparticles.
Source-Eurekalert
 MEDINDIA
MEDINDIA

 Email
Email




