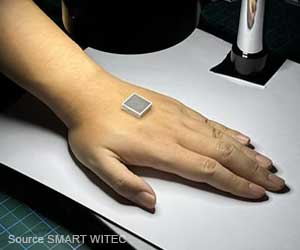
The device contains an embedded processor for data acquisition, data storage, data processing together with an accelerometer, infra-red sensor, and Bluetooth capability.
The device contains an embedded processor for data acquisition, data storage, data processing together with an accelerometer, infra-red sensor, and Bluetooth capability.
Dr. Hila Dagan, Clinical & Regulatory Affairs Manager at HealthWatch, said, "The CE Mark (number 632684) clearly recognizes the importance of the new emerging technology of continuous cardiac transmission in pursuit of improving personal health vital for the emerging m-Health industry."
Uri Amir, CEO HealthWatch, said that the CE approval adds data collection and cloud transmission to our product line offering remote 12-lead ECG monitoring saving critical time if cardiac difficulties arise.
"With this CE approval, we can start launching our MasterCaution product in Europe and ease the process of obtaining local regulatory approvals," Amir added.
 MEDINDIA
MEDINDIA
 Email
Email