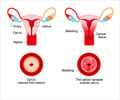
Decrease in high-grade cervical lesions in young females may be due to human papillomavirus (HPV) vaccines and changes in cancer screening recommendations.
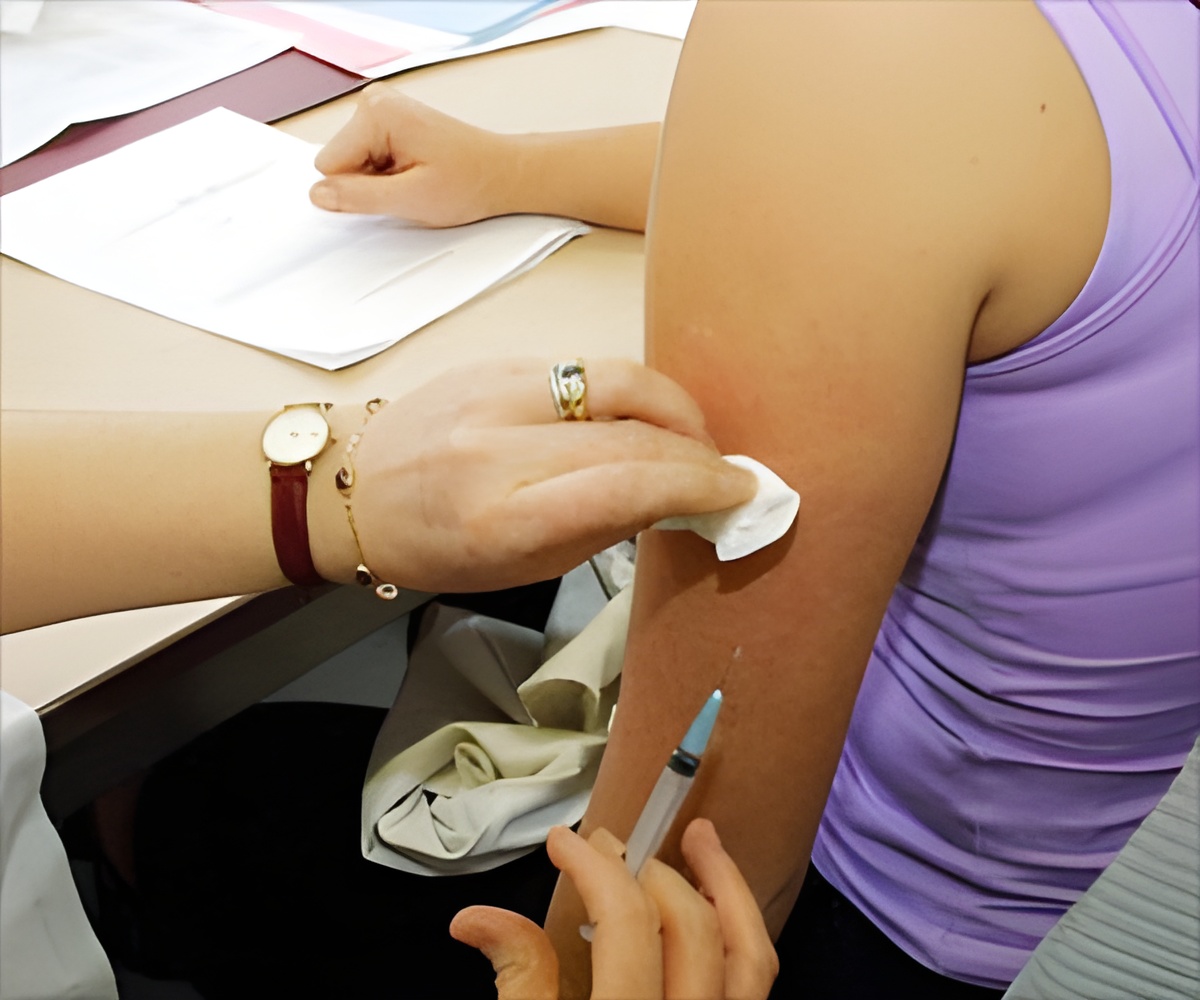
Researchers from the HPV-IMPACT Working Group evaluated the impact of the HPV vaccine in preventing cervical cancer. They found that the rate of high-grade cervical lesions which often progress to cancer (cervical intraepithelial neoplasia grade 2, 3, and adenocarcinoma in situ) had reduced in the period between 2008 to 2012 in several cities of the United States, which was the time following the introduction of the vaccine. The change was particularly noted in the age groups of 18 to 20 years, and also in the 21 to 29 years group. However, no major change was noted in the age group of 30-39 years.
Though this reduction in the pre-cancerous changes can be attributed to the impact of vaccination, the researchers caution that it could also be due to the low screening rates in the younger age groups during the same post-vaccination period. Currently, screening for cervical cancer is not routinely recommended in girls below the age of 21 years. Consequently, the researchers found a large decrease in screening among the 18 to 20 years age group, with smaller declines in the other age groups.
The US Preventative Task Force currently recommends cervical cancer screening with Pap smear in women aged 21 to 65 every 3 years. If women between the ages of 30 and 65 years want to be screened less frequently, they have the option of choosing a combination of Pap smear and HPV testing, which can be done every 5 years.
Long-term studies are required before one can say for sure that the HPV vaccine does have a significant effect in preventing cervical cancer. These studies also have to consider that the changes in screening recommendations could affect their study results as well.
Source-Medindia