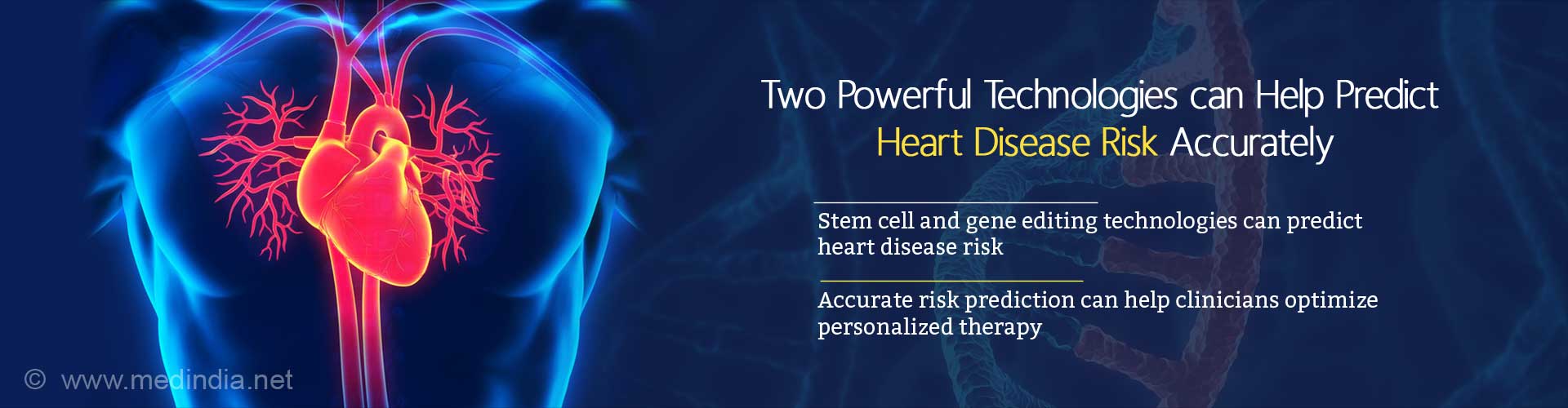
Heart disease risk can be predicted accurately by combining stem cell and gene editing technologies, as they help predict those gene variants that increase the risk of heart disease.
- Combining stem cell and gene editing technologies can help in accurately predicting heart disease-causing potential of specific gene variants
- Not all gene variants cause or increase risk of heart disease; knowing the disease-causing potential of a given gene variant helps to avoid unnecessary tests and treatment in persons who carry only a “benign” (non-disease causing) variant
- Gene variant refers to slight variations in the DNA sequences of the same gene among different individuals
TOP INSIGHT
Knowing whether a specific gene variant present in a person can cause heart disease will help clinicians to optimize therapy to suit the patient.
Aim of Study
With advances in genome and DNA sequencing, several gene variants have been discovered to be associated with certain disease conditions. However one cannot be sure whether these gene variants actually lead to disease or not.The goal of the study is to be able to characterize a given gene variant’s disease-causing potential or pathogenicity. This would help in further evaluating and treating those persons actually at risk and avoiding unnecessary treatments and undue stress to persons who may be only carrying a “benign” variant.
Details of Study
The study demonstrates for the first time, the potential benefits of combining stem cell technology to create induced pluripotent stem cells and CRISPR/Cas9-mediated genome editing technology for use as a customized risk-assessment platform to measure the disease-causing potential of a yet uncharacterized genetic variants, referred to as a "variants of uncertain significance" or VUS.- The scientists analyzed gene variants associated with hypertrophic cardiomyopathy, a disease marked by thickening of heart muscle and known to cause sudden cardiac death in athletes and young persons
- The team obtained DNA from 54 "healthy" persons without heart disease, and sequenced their DNA with a known DNA panel of 135 genes causing cardiomyopathy and congenital heart disease, associated with sudden cardiac death.
- One of the participants chosen had family members over several generations carrying an MYL3 gene variant, which is associated with hypertrophic cardiomyopathy.
- Peripheral blood mononuclear cells of the participants were isolated and these cells were engineered in the lab to form induced pluripotent stem cells (iPSCs). These were then genome edited using the CRISPR/Cas9 gene editing technology to create cells with identical genetics (isogenic iPSC lines).
- Comprehensive analysis of DNA sequence was conducted on the engineered cell lines to determine whether a given MYL3 gene variant could lead to disease.
- The DNA sequencing results unearthed 592 unique genetic variants, of which 78 percent of genetic variants were characterized as "benign," "likely benign," or a "variant of uncertain significance." However, 17 gene variants were characterized as "possibly pathogenic" or disease-causing.
"Random genetic testing will create a lot of stress for a healthy individual who may be getting echocardiograms, MRIs or medications that they may not need," Wu said. "Results from this study will help improve the interpretation and diagnostic accuracy of gene variants, especially in the era of personalized medicine and precision health. The goal is to optimize the decision making of clinicians in their choices of therapy by providing a much clearer result for the 'variant of uncertain significance' carriers."
Standard Treatment of Hypertrophic Cardiomyopathy
Generally, symptomatic patients with hypertrophic cardiomyopathy are treated based on severity of those symptoms. Asymptomatic persons are usually not treated. Treatment options include lifestyle and dietary changes, treating underlying causes that might aggravate the condition, drugs for hypertrophic cardiomyopathy, and sometimes surgery in severe cases.With this new technology, preventive interventions can be introduced in apparently healthy individuals carrying high-risk gene variant for more favorable patient outcome in the long term.
Overview of Induced Pluripotent Stem Cells and Gene Editing Technology
Induced pluripotent stem cells are increasingly being to investigate molecular and cellular changes occurring in several inherited disorders. However, one of the biggest challenges in iPSC-based research of inherited diseases hinge on discriminating between the impact of a specific mutation and the genetic background of these cells. To overcome this, scientists use the CRISPR/Cas9 gene editing technology which enables modifications of genes with a high degree of accuracy. This technique therefore enables the creation of isogenic (identical genetics) cell lines in order to focus on the disease causing ability of a specific mutationIt would be apt to conclude with the remarks of Circulation editor, Joseph A. Hill, M.D., Ph.D., chief of cardiology at UT Southwestern Medical Center in Dallas, who says, "This study combined two new powerful technologies, induced pluripotent stem cells and CRISPR-Cas9 gene editing, to model a patient's heart in a dish and to test whether those heart cells manifested signs of disease. This approach heralds a new era of in vitro disease modeling and drug testing as pivotal elements of precision medicine."
References:
- Ning Ma, Joe Zhang, Ilanit Itzhaki, Sophia L. Zhang et al., "Determining the Pathogenicity of a Genomic Variant of Uncertain Significance Using CRISPR/Cas9 and Human-Induced Pluripotent Stem Cells" Circulation (2018) https://doi.org/10.1161/CIRCULATIONAHA.117.032273
- D. G. MacArthur, T. A. Manolio, D. P. Dimmock, H. L. Rehm, et al., "Guidelines for investigating causality of sequence variants in human disease" PMC (2014) doi: 10.1038/nature13127
Source-Medindia
 MEDINDIA
MEDINDIA


 Email
Email