The injectable drug contains deoxycholic acid that destroys fat cells by breaking down the cell membrane. The drug will be commercially available in June.
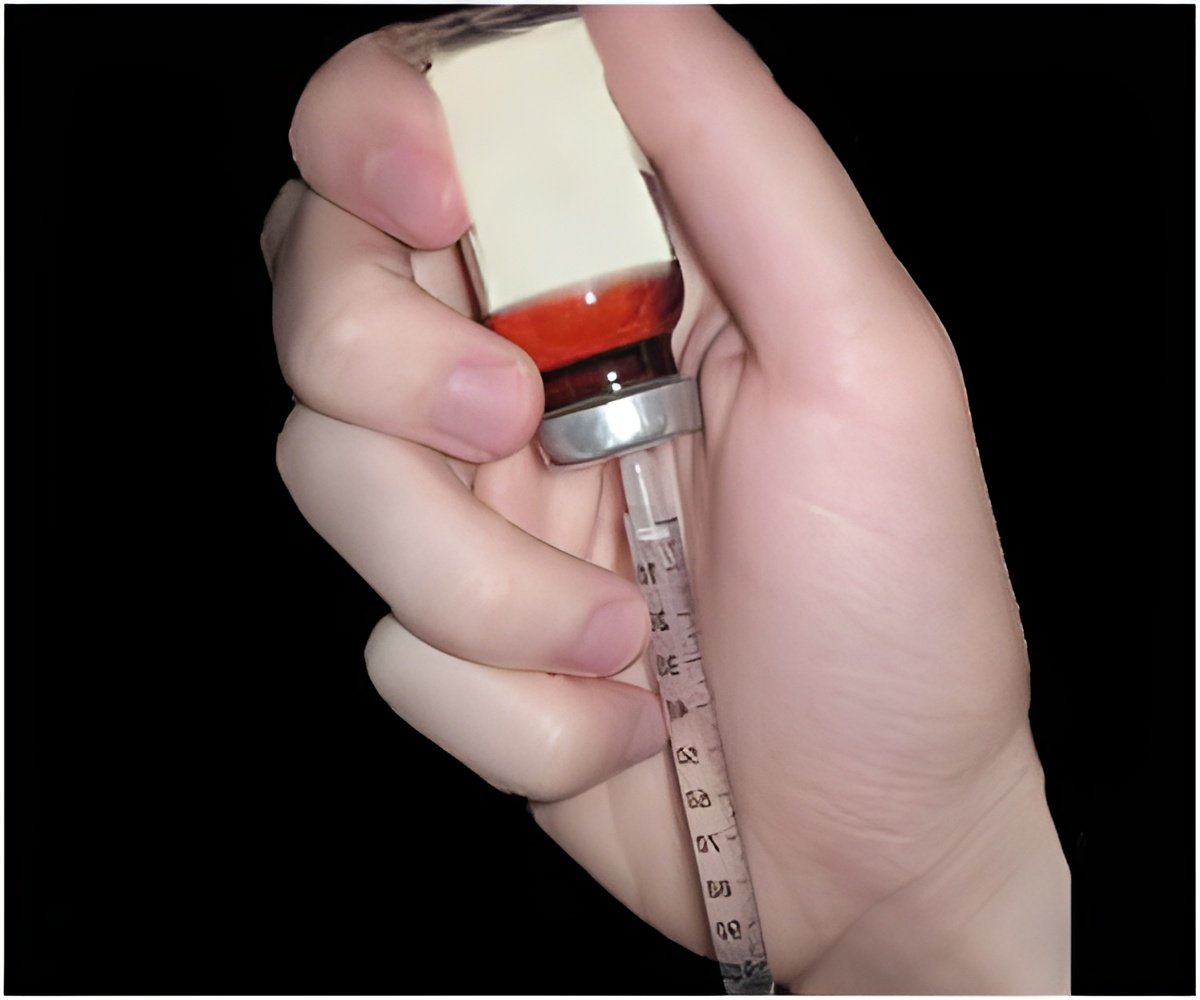
Deoxycholic acid is a naturally occurring molecule in the body that helps to absorb fat. A licensed dermatologist can inject the drug under the jawline right into the fat tissue; the procedure takes only a few minutes. The drug destroys the cell membrane of submental fat, causing it to burst. It takes two to three days to heal.
According to a survey by the American Society for Dermatologic Surgery in 2014, about 68 percent of people were concerned about excess fat under the chin and neck. Kybella will be commercially available in June.
Kybella was approved after the manufacturer submitted 19 clinical studies involving nearly 2,600 patients. The clinical tests showed that kybella worked to eliminate moderate to severe chin fat.
Currently, surgery was the only other way to eliminate double chin with traditional liposuction.
According to the FDA, the most common side effects of Kybella are swelling, bruising, pain, numbness, redness and hardness in the treatment area.
Advertisements
Source-Medindia