An oral drug for treatment of multiple sclerosis (MS) has won the nod from the Food and Drug Administration, US.
The approval is considered a valuable step forward for MS patients, who have endured frequent needle injections needed for treatment of the complex neurological condition.
Some 2.5 million people have MS worldwide, including 400,000 in the United States.
"Gilenya is the first oral drug that can slow the progression of disability and reduce the frequency and severity of symptoms in MS, offering patients an alternative to currently available injectable therapies," Russell Katz, director of the neurology products at the FDA's Center for Drug Evaluation and Research, said in a statement.
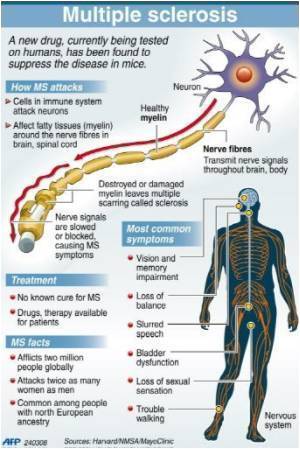
The disease can be unpredictable, with symptoms varying dramatically from one person to another. They can be mild, such as fatigue, numbness in limbs, and balance problems, or severe, including paralysis and blindness.
Novartis hailed the FDA approval and noted it came at the end of the largest-ever clinical trial program submitted to the FDA for a new MS drug.
Advertisement
"We are actively pursuing regulatory approval in Europe and the rest of the world."
Advertisement
However, the FDA warned that Gilenya carried a risk of side effects, including a decrease in heart rate for new users of the drug, as well as headache, flu, diarrhea and back pain.
The drug can also cause liver problems and make people more vulnerable to serious infections. And some cases of serious swelling behind the retinas of people taking the drug have been reported.
Still the warnings on Gilenya aren't as severe as expected.
All the same it could be hugely expensive, it is feared. A company spokeswoman told radio channel NPR that the drug would be available in the U.S. starting Oct. 4. The price tag, she says, will be based on "the value it delivers to patients, the scientific innovation it represents, and the investment Novartis has made in studies."
Health care analyst Dr. Mark Schoenebaum, with the firm International Strategy and Investment in New York, predicted that it could cost at least $30,000 per patient.
All the injected drugs cost around $35,000 a year, Schoenebaum explains, and there's no reason to think Novartis will charge much less than that.
Source-Medindia