The US Food and Drug Administration announced that it will be limiting the use of brain stents for only a small section of the patients.
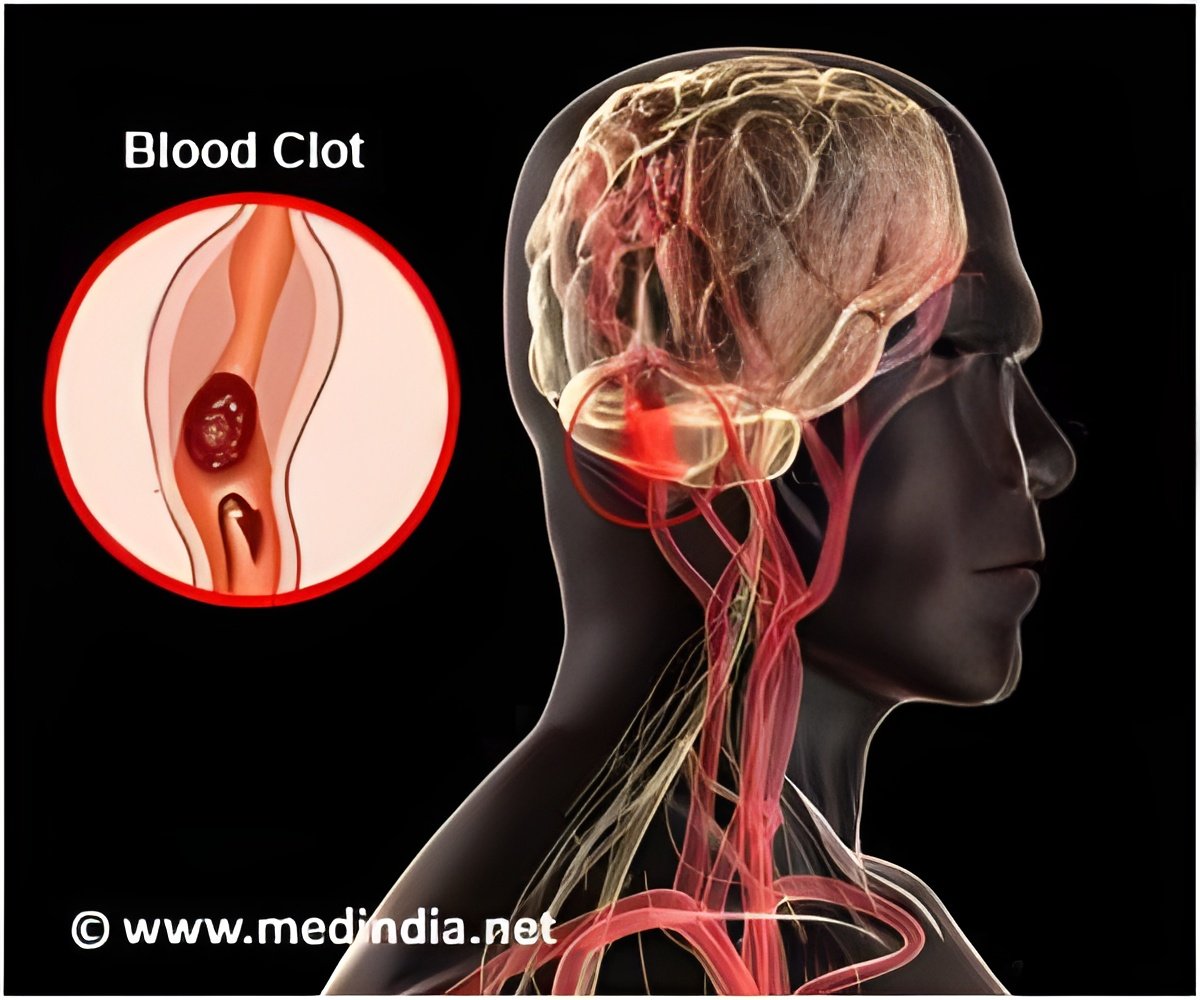
Stryker’s Wingspan stent was approved by the FDA in 2005 but the health agency said that the stent will now be used only in patients who have suffered from multiple strokes but have not experienced a stroke symptom in the last seven days.
“After careful consideration of available safety information, the FDA believes this device should remain available for this specific subgroup of patients who have exhausted other options”, the director of the FDA's center for devices, Dr Jeffrey Shuren said.
Source-Medindia
 MEDINDIA
MEDINDIA

 Email
Email