BioArchitects announced that their 3D printed, patient-specific titanium cranial/craniofacial implant has been granted 510(k) clearance by the FDA.
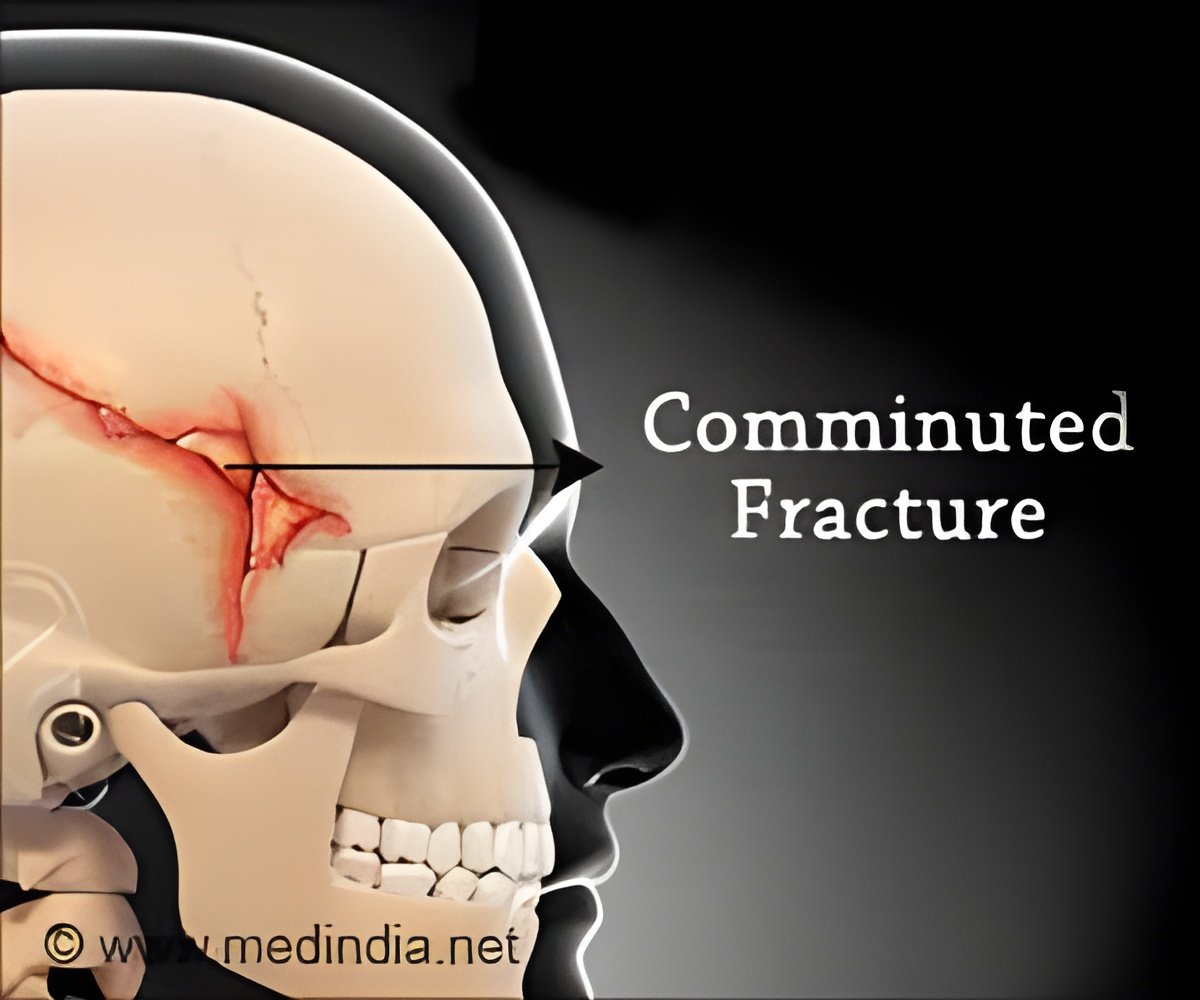
‘BioArchitects has developed patient-specific titanium cranial/craniofacial implants using 3D printing technology to reconstruct skull bones and it has been cleared by the U.S.Food and Drug Administration (FDA).’





Titanium alloy is lightweight, biocompatible and has high tensile strength. Implants made using titanium can help in the repair of bone defects resulting from trauma, disease, or congenital abnormalities. The implants are manufactured based on patient’s CT or MRI scans using Arcam's Electron Beam Melting (EBM) technology. The scan will help create a template for the repair that becomes the model from which the 3D printer produces the titanium plate. The plate will be fitted into the patients' skull with self-tapping titanium screws.
The titanium implant is suitable for hard tissue replacement as it is biologically inert and does not react with human skin. It also permits CT and MRI scanning post-surgery.
“We are extremely proud to contribute to what we consider another major advance in the trend toward personalized medicine. We believe that this is yet another step toward what will ultimately become the new standard of care," said Mark Ulrich, CEO of BioArchitects USA.
Source-Medindia