Researchers at EPFL have made use of bioengineering to significantly improve the efficacy of clinical growth factors.
A group led by Jeffrey A. Hubbell at EPFL has found a way to vastly improve the efficiency of growth factors, while keeping their usage at low doses. The group screened 25 growth factors against six key proteins of the extracellular matrix – the supporting structure that surrounds organs and tissues in the body and is heavily associated with mediating the function of growth factors. Physiologically, the growth factors interact with these proteins to stimulate cell growth in damaged tissues by activation of receptors. In the screening test, the 25 growth factors bound to the six proteins with varying strengths, allowing the researchers to select one growth factor (PIGF-2) that showed the strongest binding across all six proteins.
By analyzing the sequence of the growth factor, the scientists isolated a 22-amino acid section that is responsible for the powerful binding of PIGF-2 to extracellular matrix proteins. By fusing that sequence to three growth factors they were able to increase their binding affinity by 2- to 100-fold, which could reduce the need for higher doses in the future. In addition, the bioengineered growth factors showed that they could mimic interactions in the formation of a blood clot, which would be additional beneficial to wound-healing.
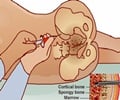
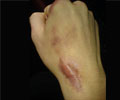
The group also tested low-dose topical application of growth factors on diabetic mice, which are a common model for impaired wound healing. Compared to their unmodified counterparts, the growth factors containing the PIGF-2 sequence resulted in much faster wound closing and production of granulation tissue, and also led to a more pronounced new blood vessel formation, which is essential in sustaining the latter. The researchers also saw similar effects in bone repair, with the engineered growth factors showing a much higher deposition of bone tissue in rats with skull defects. Finally, they were able to show that the clinical side-effects of one particular growth factor could be alleviated by replacing it with its bioengineered counterpart.
The results show that a relatively simple modification can greatly improve the clinical use of growth factors, by making them more efficient, cost-effective and safe. The group is now fusing the PIGF-2 sequence to additional growth factors, which they can do in a virtually plug-and-play fashion. "Evolution has provided a close interaction between the extracellular matrix and growth factors", says Hubbell. "By re-engineering the molecules, we are able to exploit that interaction and open the way for clinical translation, turning these molecules into useful drugs." The researchers are now planning to extend their studies to larger animal models and eventually begin preliminary human trials.
Advertisement