‘2-hydroxypropyl-β-cyclodextrin (HPβCD) does not cross the blood-brain barrier in therapeutically relevant amounts to address the neurological manifestations of Niemann-Pick Disease Type C1.’
Tweet it Now
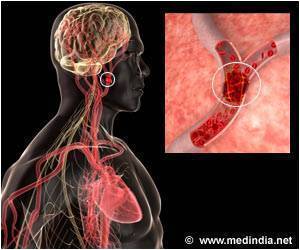
NPC1 is an autosomal-recessive, rare lysosomal storage characterized by progressively debilitating and ultimately fatal neurological manifestations. Clinical trials of different HPβCD agents are currently underway and the route of administration is an important point of consideration for the anticipated results of these trials with regard to safety, tolerability and efficacy in the NPC1 population. Cyclodextrins are complex mixtures of different chemical species and different cyclodextrin products are therefore not the same as one another. When administering drugs directly to the CNS, it is important that the agent being administered is highly purified and well characterized to avoid introducing any contaminants and/or unknown agents that could adversely affect neurological development and/or function. Currently, VTS-270 is the only specific and well-characterized mixture of HPβCD, with a tightly controlled molar substitution specification and a defined molecular "fingerprint" of the different chemical species present in the mixture based on Kleptose® HPB (Roquette Pharma, France).
Source-Eurekalert