Two biological mechanisms have been identified by researchers from Boston and at Johns Hopkins as the major cause of protein citrullination which triggers the immune system.
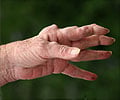
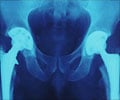
Researchers say such disruptions in cell membrane integrity produce enzyme-activating imbalances of calcium ions. The activated enzymes, called PADs, are known to citrullinate specific, protein-forming amino acids in hand, knee, foot, and elbow joints, setting off the inflammatory immune response that is rheumatoid arthritis' hallmark.
According to researchers, the latest study findings open up a whole new field of study into rheumatoid arthritis' origin and into the mechanisms that might start the disease process.
"For a long time, it has been suspected that immune pathways activated in response to pathogens play a role in initiating immune responses in rheumatoid arthritis," says senior study investigator and rheumatologist Felipe Andrade, M.D., Ph.D.
"This study offers a renewed framework to support and to study this hypothesis, and provides novel targets that may be amenable for monitoring and treatment," says Andrade, an assistant professor at the Johns Hopkins University School of Medicine.
In the study, researchers initially identified that cells obtained from the joints of patients with rheumatoid arthritis contain a unique pattern of citrullination, which they termed cellular hypercitrullination. Lab experiments showed that this pattern was not reproduced by cell death and cell activating pathways previously thought to be responsible for the process. Some stimuli induced citrullination, but none induced the hypercitrullination seen in the disease.
Advertisement
Of 20 stimuli studied, all known to promote inflammatory pathways active in the rheumatoid joint, only two, perforin and MAC, reproduced the hypercitrullination detected in rheumatoid arthritis patients.
Advertisement
"Our results suggest that inhibiting these pathways may have real benefit in patients and represent an important field of study for investigating new and alternative treatments," says Andrade.
Andrade and his team next plan to determine the mechanisms responsible for abnormal activation of the perforin and MAC pathways in rheumatoid joints. He says this may lead to the identification of the earliest components that initiate the disease process.
The team also plans to define novel biomarkers to monitor the citrullination activity induced by these pathways in people with rheumatoid arthritis, with the goal of matching levels to disease
progression and response to treatment.
Funding support for this study was provided by the Dana Foundation Scholars Program in Human Immunology, the Donald B. and Dorothy L. Stabler Foundation, Ira T. Fine Discovery Fund, the Mackley Fund from Sibley Memorial Hospital, the Johns Hopkins Arthritis Discover Fund and the National Institutes of Health. Corresponding grant numbers are P30-AR053503, 1R21HL112586-01, HHSN268201000032C and R37-DE-12354.
Besides Andrade, other Johns Hopkins researchers involved in this study were lead investigator Violeta Romero, Ph.D.; Justyna Fert-Bober, Ph.D.; Erika Darrah, Ph.D.; Uzma Haque, M.D.; Jennifer Van Eyk, Ph.D.; and Antony Rosen, Johns Hopkins' director of rheumatology and Mary Betty Stevens Professor. Additional research assistance was provided by Peter Nigrovic, M.D.; and David M. Lee, M.D., Ph.D., at Harvard University.
Johns Hopkins Medicine (JHM), headquartered in Baltimore, Maryland, is a $6.7 billion integrated global health enterprise and one of the leading academic health care systems in the United States. JHM unites physicians and scientists of the Johns Hopkins University School of Medicine with the organizations, health professionals and facilities of The Johns Hopkins Hospital and Health System. JHM's vision, "Together, we will deliver the promise of medicine," is supported by its mission to improve the health of the community and the world by setting the standard of excellence in medical education, research and clinical care. Diverse and inclusive, JHM educates medical students, scientists, health care professionals and the public; conducts biomedical research; and provides patient-centered medicine to prevent, diagnose and treat human illness. JHM operates six academic and community hospitals, four suburban health care and surgery centers, and more than 35 Johns Hopkins Community Physicians sites. The Johns Hopkins Hospital, opened in 1889, was ranked number one in the nation for 21 years in a row by U.S. News & World Report. For more information about Johns Hopkins Medicine, its research, education and clinical programs, and for the latest health, science and research news, visit
Source-Newswise