Lutetium Lu 177 Dotatate Medication Information
Discover comprehensive details about Lutetium Lu 177 Dotatate, including its pronunciation, uses, dosage instructions, indications, and guidelines on how and when to take it or avoid it.
The updated prescription information covers potential side effects, precautions, warnings, and storage recommendations.
Additionally, explore the Lutetium Lu 177 Dotatate brands available in India and internationally, along with pricing information. For personalized advice, consult your healthcare provider.
Generic Name : Lutetium Lu 177 Dotatate Therapeutic Classification : Radiopharmaceutical, AntineoplasticBrand Names or Trade Names of Lutetium Lu 177 Dotatate
India :
Lutathera
Overview of Lutetium Lu 177 Dotatate
• Lutetium Lu 177 dotatate injection for intravenous use was approved by the US FDA in January 2018 for the treatment of certain gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in adults.Why is Lutetium Lu 177 Dotatate Prescribed? (Indications)
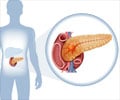
Lutetium Lu 177 dotatate is a somatostatin analog radioactive drug or a radiopharmaceutical which is used for treating adult patients with somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).These tumors arise from specialized cells of the digestive tract or the pancreas and may secrete substances like serotonin, gastrin, insulin, vasoactive intestinal polypeptide and glucagon.
Lutetium Lu 177 dotatate works by attaching to a specific type of protein on the tumor cells called somatostatin receptors. The attachment allows the compound to enter the cells and emit radiation resulting in cell damage.
When should Lutetium Lu 177 Dotatate not be taken? (Contraindications)
Lutetium Lu 177 dotatate should not be used in the following conditions:• Allergy to lutetium Lu 177 dotatate
• Pregnancy and breastfeeding
• Severe liver or kidney disorder
• Children
What is the dosage of Lutetium Lu 177 Dotatate?
• The recommended dosage of lutetium Lu 177 dotatate in adults is 7.4 GBq (200 mCi) administered every eight weeks for a total of 4 doses.• Dose modifications are recommended in patients with adverse reactions either by reducing the dose or if necessary, by permanently discontinuing the treatment.
How should Lutetium Lu 177 Dotatate be taken?
• Lutetium Lu 177 dotatate comes as a liquid which should be administered intravenously using a particular technique by trained healthcare personnel.• Lutetium Lu 177 dotatate is slowly infused along with 0.9% sodium chloride using specific precautions and technique. It is necessary to flush 25 ml of 0.9% sodium chloride following the lutetium Lu 177 dotatate infusion.
• An infusion of amino acid solution containing L-lysine and L-arginine should be administered beginning 30 minutes before and continuing for at least 3 hours after lutetium Lu 177 dotatate administration. Anti-emetics (medications to prevent vomiting) should be administered 30 minutes before administering the amino acid solution.
• Aseptic technique and appropriate safety measures such as wearing waterproof gloves radiation shielding are necessary while administering lutetium Lu 177 dotatate. To reduce the exposure to radiation, tongs can be used while handling the vial.
• Long-acting octreotide 30mg should be administered intramuscularly between 4 to 24 hours after each lutetium Lu 177 dotatate administration, while short-acting octreotide can be administered in between as needed. Somatostatin analogues like octreotide reduce the secretion by the tumor and thereby reduce symptoms like diarrhea and flushing caused by the tumor.
• However, a long-acting somatostatin analogue should not be used at least four weeks before, or short-acting octreotide should not be administered at least 24 hours before a dose of lutetium Lu 177 dotatate, since it could interfere with the treatment.
• Once the treatment with lutetium Lu 177 dotatate is completed, the patient should receive long-acting octreotide 30 mg intramuscularly every 4 weeks for up to 18 months since the start of treatment. The treatment with long-acting octreotide can be stopped early in case the disease continues to progress.
What are the warnings and precautions for Lutetium Lu 177 Dotatate?
• Women of childbearing age should undergo a pregnancy test before beginning the treatment and treatment should be started only if the test shows a negative result. Both women and men should adopt effective contraceptive methods during the treatment which should be continued after the treatment for at least 7 months in women and 4 months in men.• Lutetium Lu 177 dotatate should be administered only by the government approved or licensed physicians who are qualified with adequate training and skill in handling the radiopharmaceutical safely.
• • Confirm the radioactivity in the vial before and after the administration of the medication with a dose calibrator.
• The vial should be inspected for the presence of any particulate matter or discoloration under a shielded screen before administration and should be discarded if these are present.
• Patients are advised to urinate frequently during the treatment. The serum creatinine levels and creatinine clearance should be monitored to detect any toxic effects on the kidneys.
• • Monitor blood cell counts regularly to detect the presence of myelodysplastic syndrome (a group of conditions in which the production of blood cells by the bone marrow are affected), acute leukemia (cancer of white blood cells) or myelosuppression (reduced production of blood cells by the bone marrow) which can be controlled by either reducing the dose or discontinuing the treatment permanently.
• Serum albumin, bilirubin, and transaminase enzyme levels should be monitored to check for liver damage. A neuroendocrine hormonal crisis with bronchoconstriction, flushing, decreased blood pressure, and diarrhea could occur during treatment with lutetium Lu 177 dotatate. It can be managed with the administration of the fluids or electrolytes, corticosteroids, and somatostatin analogs.
• Lutetium Lu 177 dotatate may cause infertility in males and females. Therefore, men or women who wish to bear children after the treatment are given the option to store their sperms or eggs in a sperm or egg bank before commencing the treatment.
What are the side effects of Lutetium Lu 177 Dotatate?
Gastrointestinal: Nausea, vomiting, diarrhea, stomach pain, constipation, loss of appetiteBlood: Reduced or altered counts of red blood cells, white blood cells, platelets, acute leukemia
Cardiovascular: Irregular heartbeat (atrial fibrillation), swelling of the legs and ankles, increased blood pressure
Nervous system:Dizziness, headache, taste disturbances, anxiety
Musculoskeletal: Muscle pain, back pain, pain in the legs, hands, and neck
Kidneys and Urinary Tract: Kidney dysfunction, kidney failure, urinary symptoms due to damage to the urinary tract which may include pain, urinary incontinence, urgency while passing urine and/or increased frequency
Metabolic abnormalities: Increased blood level of uric acid or sodium, low blood calcium levels, fluctuations in blood sugar or potassium level.
Others: Fatigue, fever, cough, liver dysfunction (especially in those with spread of the cancer to the liver), increased risk of cancer, hair loss, neuroendocrine hormonal crisis with symptoms that may include breathlessness, flushing, decreased blood pressure and diarrhea
What are the other precautions for Lutetium Lu 177 Dotatate?
• Lutetium 177 dotatate should not be given as an intravenous bolus and is necessary to administer it according to the instructions provided.• Unused medicinal products or medical waste from the treatment should be disposed of in accordance with the rules laid down by the local law and regulations authority.
What are the Drug Interactions of Lutetium Lu 177 Dotatate?
• Somatostatin analogs when co-administered with lutetium Lu 177 dotatate results in reduced therapeutic activity and should not be taken together.• Patients should inform the physician about the prescription or non-prescription drugs that they may be taking to avoid serious drug interactions.
What are the storage conditions for Lutetium Lu 177 Dotatate?
• Store at room temperature below 25° C.• The solution should be discarded as per the safety standards after 72 hours of opening.
 MEDINDIA
MEDINDIA
 Email
Email